Does Aluminium Rust? Passivation, Corrosion, And Real Fixes

Does Aluminium Rust? The Fast, Science-Backed Answer
Quick answer at a glance
Quick answer: No, aluminum doesn’t rust like iron; it forms a thin, protective oxide layer that resists further corrosion.
What rust really is
Let’s start with the basics. Rust is a specific type of corrosion that only happens to iron and its alloys (like steel). When iron reacts with oxygen and water, it forms iron oxide—a reddish-brown, flaky substance we all recognize as rust. This rust layer is weak and crumbly, so it falls off and exposes fresh metal, which keeps the process going.
Corrosion, on the other hand, is a broader term. It’s the gradual breakdown of any metal due to chemical reactions with its environment. So, while all rust is corrosion, not all corrosion is rust. This is where aluminum stands out.
Why aluminum behaves differently
Will aluminium rust if you leave it outside or expose it to water? The answer is no—aluminum can’t form iron oxide because it doesn’t contain iron. Instead, when exposed to air or moisture, aluminum reacts quickly with oxygen to form aluminum oxide (Al2O3). This oxide layer is:
- Extremely thin and nearly invisible, so the metal keeps its appearance
- Strongly bonded to the aluminum surface, unlike the flaky layers of rust on steel
- Self-healing—if scratched, a new layer forms almost instantly as long as oxygen is present
This is why, in most everyday situations, you’ll notice that aluminum rust simply isn’t a thing. But does that mean aluminum is invincible? Not quite. Under certain conditions—like exposure to saltwater or harsh chemicals—aluminum can still corrode, though the process and results look very different from rust on steel. For example, you might see a white, chalky residue or small pits instead of red flakes.
Key takeaways you can trust
- Aluminum doesn’t rust, but it can corrode under some conditions
- Its oxide layer is self-limiting and generally protects the metal beneath
- Harsh environments or poor design can still cause damage—so maintenance matters
Still wondering, “do aluminum rust” or “can aluminum rust”? The science is clear: while aluminum doesn’t rust, understanding its unique corrosion process is key to making smart material choices.
What’s next in this guide?
In the sections that follow, you’ll get a deeper look at:
- The chemistry behind aluminum’s protective oxide layer
- Common types of corrosion in aluminum and how to spot them
- How different alloys and environments affect corrosion risk
- Practical tips for maintenance and repair
- Testing standards and how to choose the right material for your project
By the end, you’ll be able to answer not just “does aluminium rust,” but also how to keep your aluminum parts strong and looking their best for years to come.

How Passivation Protects Aluminum
How the oxide film forms
Ever wondered what really happens when you leave a piece of aluminum exposed to air or water? The answer lies in a simple, but powerful, chemical reaction: as soon as bare aluminum meets oxygen, it reacts instantly to form a thin layer of aluminum oxide (Al2O3). This process is called oxidation on metal, and it’s what gives aluminum its impressive resistance to further attack. Unlike iron, which forms flaky rust, aluminum’s oxide layer is tightly bonded to the surface and acts as a barrier to moisture, air, and most chemicals.
- Thickness: The natural oxide film is extremely thin—often just a few nanometers—but it’s continuous and covers every exposed area.
- Appearance: It’s usually clear, so you won’t see it, but in some cases, it can give a dull gray sheen to the metal.
- Adhesion: This layer sticks so well that it rarely flakes off, unlike the rust you see on steel.
- Self-healing: If the film is scratched or damaged, it rapidly reforms in the presence of oxygen.
Self-healing protection explained
Think of the oxide layer as an ultra-thin, invisible raincoat for aluminum. If you scratch it, the metal underneath immediately reacts with air and “reseals” itself—almost like a magic raincoat that repairs holes as soon as they appear. This is why aluminum oxidation is generally protective, not destructive. In contrast, when iron rusts, the rust layer expands and flakes off, exposing even more fresh metal to corrosion. That’s the key difference in the corrosion vs rust story: aluminum’s oxide stays put and shields the metal, while rust just keeps eating away at iron.
Self-healing does not mean invincible. Certain environments—like saltwater, trapped moisture, or contact with other metals—can still break down the oxide barrier and lead to corrosion in aluminum.
When passivation breaks down
While the natural oxide film on aluminum is tough, it’s not unbreakable. Here’s when trouble can start:
- Chlorides (like seawater): Salt can penetrate and disrupt the oxide layer, causing pitting and localized attack.
- Stagnant moisture: Water trapped in crevices or under debris may prevent the oxide from reforming, leading to corrosion in aluminum.
- Dissimilar metals: Contact with copper, steel, or other metals can set up galvanic reactions that accelerate corrosion.
- Contaminants: Oils, dirt, or industrial pollutants can interfere with the protective film.
- Poor design: Features that trap water or prevent airflow can increase the risk of oxidation on aluminium and corrosion.
It’s also worth noting that while passivation is the natural process that protects aluminum, it can be enhanced with treatments like anodizing. Anodizing deliberately thickens the oxide layer, making it even more durable and resistant to wear and corrosion (source).
So, does aluminum oxidize? Absolutely—but in most cases, this oxidation on metal works in your favor. However, if the environment is harsh or the design traps moisture, even oxidized aluminum can suffer damage. Up next, you’ll learn how to recognize different types of corrosion in aluminum and what warning signs to watch for.
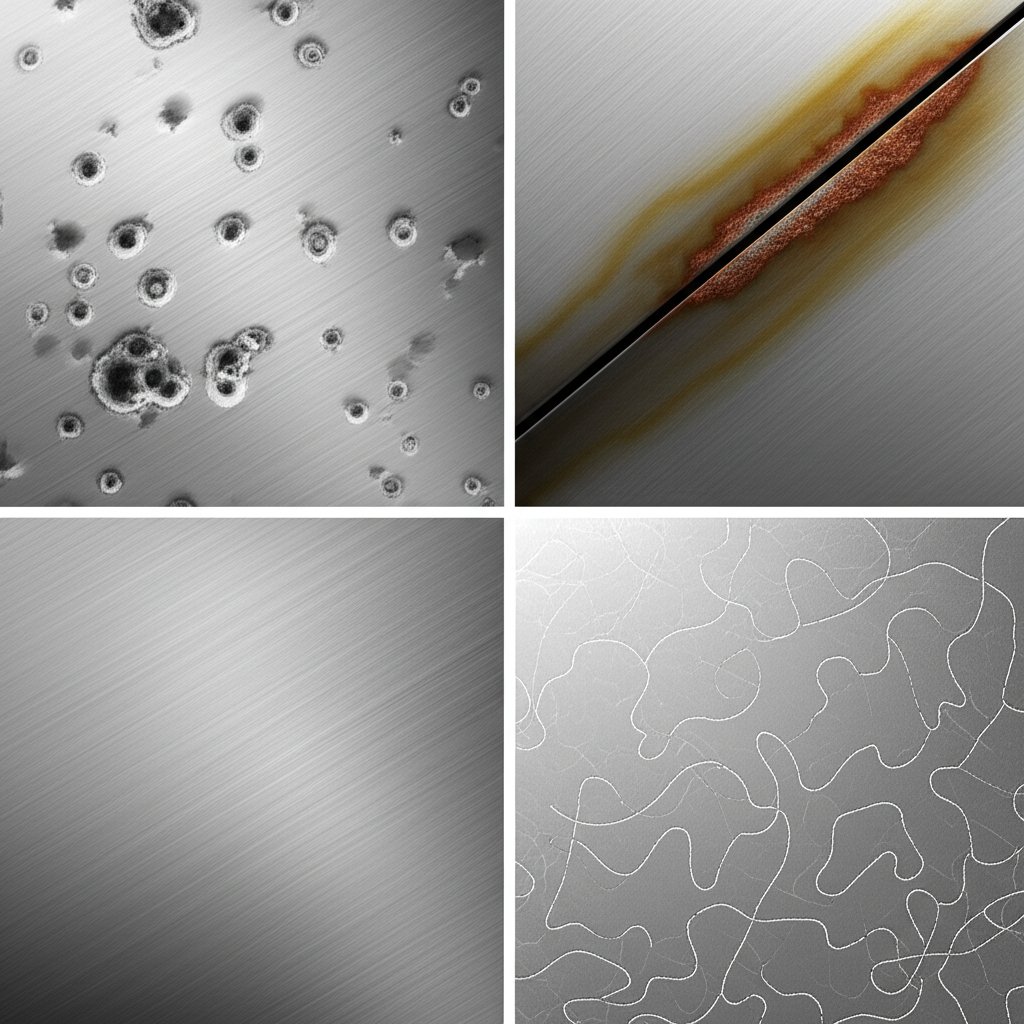
Recognizing Aluminum Corrosion
Pitting and Crevice Attack
- Appearance: Small, sharp-edged holes or pinhole craters—sometimes clustered—often with a white, powdery residue around them. These pits may be shallow or, over time, deepen and grow.
- Where it occurs: Most common in areas exposed to salt (like coastal air or road de-icing), or where moisture collects—think window frames, boat fittings, or anywhere water sits on the surface. Crevice corrosion appears in tight gaps, such as under gaskets, between overlapping panels, or beneath fastener heads.
- What to do next: Inspect areas that stay damp or collect debris. Remove gaskets or covers to check hidden surfaces. Clean and dry affected spots, and consider applying sealant or improving drainage to prevent recurrence. For persistent pitting, more robust coatings or switching to a more resistant alloy may be necessary.
Galvanic Problems with Stainless Steel and Other Metals
- Appearance: Corrosion is often concentrated right around the joint where aluminum meets stainless steel or carbon steel. You might see deep pitting, surface etching, or even rapid material loss—sometimes with white or grayish corrosion products. This is classic aluminum steel corrosion or aluminium stainless steel corrosion.
- Where it occurs: At fastener sites (bolts, screws, rivets), railings, brackets, or any assembly where stainless steel and aluminum are directly connected—especially in wet or salty environments. Stainless steel to aluminum corrosion is most aggressive when water (especially saltwater) bridges the two metals.
- What to do next: Check for insulation between metals—such as plastic washers or coatings. If you see corrosion, break the electrical contact by adding isolators or re-coating the materials. In severe cases, replace affected parts and redesign the joint to minimize direct contact.
Galvanic pairs—like stainless fasteners in bare aluminum—can destroy aluminum around the joint if not electrically isolated. Always use insulating washers, gaskets, or coatings to break the circuit.
Filiform and Uniform Attack
- Appearance: Filiform corrosion looks like fine, thread-like trails or "worm tracks" under paint or coatings, often starting at scratches or coating defects. Uniform corrosion appears as a general dulling, with a powdery white or gray film covering large areas, and a gradual thinning of the metal.
- Where it occurs: Filiform is common under painted or powder-coated surfaces, especially where the coating is damaged and humidity is high. Uniform corrosion happens in environments with persistent acidity or alkalinity, or where the protective oxide layer is compromised everywhere (for example, industrial plants or chemical processing).
- What to do next: For filiform, repair damaged coatings and keep surfaces clean and dry. For uniform attack, identify and address the chemical source, and consider upgrading to a more durable finish or alloy.
Stress Corrosion Cracking (SCC) Overview
- Appearance: SCC is subtle—look for fine, branched cracks, often difficult to see with the naked eye. These cracks may start at stressed corners, welds, or notches. Unlike other types, SCC is less about visible corrosion products and more about hidden structural weakness.
- Where it occurs: In high-strength aluminum alloys under constant tensile stress, especially in humid or marine environments. Watch for it in load-bearing frames, pressure vessels, or aircraft parts.
- What to do next: If you suspect SCC, reduce stress on the part, improve environmental controls, and consult a specialist. Cracked components usually require replacement, not just surface repair.
Other Notable Corrosion Types
- Exfoliation: Flaky, layered separation of the surface, often in rolled or extruded products—signals severe internal damage.
- Deposition (from heavy metals): Localized pitting where copper or other heavy metal ions have deposited onto aluminum—watch for this near plumbing or in industrial water systems.
So, what does aluminum corrosion look like? It ranges from tiny pits and white powder to deep craters at metal joints or even invisible cracks that threaten structural safety. The types of aluminum corrosion you’ll encounter depend on the environment, design, and choice of materials—especially when stainless steel and aluminum reaction is possible.
Next, we’ll explore how different aluminum alloy families stack up against these threats, so you can make smarter choices for your application.
Alloy Families and Corrosion Resistance
1xxx to 7xxx in Plain Language: What Makes Each Family Unique?
When you’re deciding which aluminum to use, it’s not just about strength or price—corrosion resistance can make or break your project. But with hundreds of grades out there, how do you cut through the confusion? Let’s break down the major aluminum alloy families, focusing on how each stands up to corrosion, and why some are better suited for tough environments than others.
| Alloy Family | Typical Uses | Relative Pitting Risk | Stress Corrosion Cracking (SCC) Concern | Galvanic Considerations |
|---|---|---|---|---|
| 1xxx (Pure Aluminum) | Electrical conductors, chemical tanks, enclosures | Very low | Very low | Low; but still vulnerable if paired with dissimilar metals |
| 3xxx (Manganese) | Heat exchangers, cookware, automotive trim, cans | Low | Low | Moderate; galvanic attack possible in saltwater |
| 5xxx (Magnesium) | Marine structures, shipbuilding, storage tanks, bridges | Very low (especially marine grades like 5083, 5086, 5456) | Low to moderate (some grades more sensitive in high-stress, high-temp) | Moderate; still requires isolation from steel or copper in wet settings |
| 6xxx (Magnesium-Silicon) | Building frames, railings, marine fittings, extrusions | Moderate (good, but less than 5xxx in saltwater) | Moderate (can be susceptible under certain loads and environments) | Moderate; galvanic risk around fasteners |
| 2xxx (Copper) | Aerospace, high-strength structural parts | High (copper content increases corrosion risk) | High (especially SCC in aggressive environments) | High; needs careful design and extra protection |
| 7xxx (Zinc-Mg-Cu) | Aerospace, critical load-bearing components | Moderate to high (varies by copper content) | High (SCC is a major concern in some grades) | High; avoid direct contact with steel or stainless |
Non-Heat Treatable vs. Heat Treatable Alloys: Why It Matters
Here’s a quick tip: Alloys in the 1xxx, 3xxx, and 5xxx series are non-heat treatable, meaning their strength comes from cold working, not heat. These grades, especially the 5xxx family, are known for excellent aluminum corrosion resistance—even in marine environments. That’s why you’ll see 5083 or 5086 on boat hulls and offshore structures.
Heat-treatable alloys (2xxx, 6xxx, 7xxx) are prized for their high strength. However, this can come at the cost of corrosion resistance. For example, 2xxx (copper-rich) alloys are strong but can aluminium corrode much faster, especially in damp or salty air. 6xxx alloys strike a balance—decent strength, good workability, and moderate corrosion resistance—making them a go-to for architectural and light marine hardware. 7xxx alloys (zinc, magnesium, copper) are aerospace favorites, but some grades can be vulnerable to both pitting and stress corrosion cracking, especially if copper is present.
Notes on Marine and Structural Choices
If you’re asking, “Is aluminum corrosion resistant enough for saltwater?” focus on the 5xxx series. Alloys like 5083, 5086, and 5456 are engineered for marine punishment—they form a robust oxide barrier and lose metal at a fraction of the rate of standard grades. 6xxx alloys (like 6061) are widely used for marine fittings and frames, but if you need the best aluminium corrosion resistance, 5xxx is often the safer bet (source).
On the flip side, 2xxx and some 7xxx alloys demand careful design and robust coatings if they’ll face wet or aggressive environments. Does aluminium corrode in fresh air? Rarely. But can aluminum corrode rapidly in salt, acid, or when paired with steel? Absolutely—especially for the less-resistant families. Always consider isolation, protective coatings, and regular inspection for these grades.
Key reminder: The specific temper (heat treatment) and variant of each alloy can shift its corrosion resistance. Always check datasheets and standards for your exact grade—what works for one 6xxx alloy may not work for another.
Understanding the corrosion resistance of aluminum alloys helps you match the right material to the right job, reducing surprises down the road. Next, we’ll see how finishes and fabrication choices can further boost your aluminum’s defense against the elements.

Finishes and Fabrication Choices That Prevent Aluminum Corrosion
Finishes That Work: Anodizing and Powder Coating
When you want to make sure your aluminum project stands the test of time, the finish you choose is just as important as the alloy itself. Sounds complex? It doesn’t have to be. Two of the most effective aluminum corrosion protection methods are anodizing and powder coating—each with its own strengths for different environments and design goals.
- Anodizing: This process thickens and hardens the natural oxide layer on aluminum, making it even more resistant to corrosion and wear. It’s great for applications where you want a durable, metallic appearance and tight dimensional tolerances. Anodized aluminum is also less likely to suffer from aluminium rust proof failures in harsh settings, and it’s a top pick for structural and architectural uses.
- Powder Coating: If you want vibrant colors, unique textures, or extra protection from the elements, powder coating is your go-to. This finish creates a thick, uniform barrier that shields aluminum from moisture, chemicals, and UV rays. It’s especially popular for outdoor equipment, architectural trim, and consumer products where both looks and durability matter.
Both finishes offer excellent aluminium corrosion protection, but your choice depends on design needs, exposure, and budget. Remember: no finish can compensate for poor surface prep or missed edges—proper cleaning and full coverage are essential for lasting results.
Design for Drainage, Isolation, and Airflow
Even the best aluminium protective coating can be undermined by water traps, hidden joints, or metal-to-metal contact. Imagine rainwater pooling inside a window frame or two metals touching without insulation—these are classic setups for aluminium steel corrosion or aluminum and steel reaction. Smart design choices can make all the difference:
- Isolate dissimilar metals: Use nylon washers, plastic gaskets, or non-conductive sealants between aluminum and steel or stainless steel parts to prevent galvanic corrosion. Direct aluminum and steel reaction can accelerate damage, especially in wet conditions.
- Promote drainage: Add drain holes wherever water might collect—window sills, hollow rails, or enclosure bottoms. Good drainage reduces the risk of steel aluminium corrosion and keeps the oxide layer healthy.
- Encourage airflow: Design assemblies to allow air circulation, which helps surfaces dry out quickly and limits corrosion risk.
- Avoid crevices: Minimize tight gaps where moisture can get trapped. Overlapping joints, unsealed seams, and tight corners are hotspots for aluminium steel corrosion.
- Specify smooth radii: Sharp bends or corners are more likely to crack or lose finish coverage—use generous radii for better protection.
- Seal cut edges: After cutting or machining, always seal exposed edges with touch-up finish or sealant to maintain a continuous protective barrier.
Fastener and Joint Practices That Reduce Risk
Ever noticed how corrosion often starts around screws, bolts, or welds? That’s because joints are prime sites for water ingress and galvanic activity. Here’s how to keep these weak points strong:
- Choose compatible fasteners: Select aluminum or coated fasteners for aluminum assemblies, or ensure stainless steel fasteners are electrically isolated from aluminum parts.
- Apply sealants: Use non-conductive sealants in joints and under fastener heads to block moisture and prevent steel aluminium corrosion.
- Weld with care: If welding is required, ensure welds are finished and coated to prevent crevice corrosion at heat-affected zones.
- Inspect regularly: Schedule routine checks for early signs of aluminum and steel corrosion, especially at joints and fastener locations.
Isolation + drainage + finish quality = longest life.
Bringing It All Together: From Design to Fabrication
For the best aluminum corrosion protection, it’s not just about picking the right finish or alloy—it’s about integrating smart design and fabrication choices from the start. Need help turning these principles into reality? Partnering with an ISO 9001 certified cnc machining services that offers anodizing, powder coating, and precise cutting and bending can ensure your parts have minimal crevices, robust finishes, and proper isolation for every joint. This approach is key to preventing aluminium steel corrosion and maximizing the lifespan of your build.
| Checklist: Aluminum Corrosion Protection Best Practices |
|---|
|
By combining the right finish, thoughtful design, and professional fabrication, you’ll achieve aluminium rust proof performance and keep aluminum and steel corrosion at bay. Up next, we’ll dive into how different environments—from salty coasts to industrial sites—affect your aluminum’s longevity and what you can do to stay ahead of the risks.
How Exposure Influences Aluminum Corrosion
Marine and Coastal Exposure
-
Risk factors:
- Chloride ions from saltwater aggressively attack the protective oxide layer, leading to pitting corrosion.
- Constant humidity and salt spray increase the risk, especially for aluminum in direct contact with water or in splash zones.
-
Mitigations:
- Choose marine-grade alloys (such as 5xxx or 6xxx series) specifically engineered to resist saltwater corrosion.
- Apply anodizing or high-quality powder coating to reinforce the oxide barrier.
- Use sealed or isolated fasteners to prevent galvanic reactions with other metals.
- Design for natural drainage and rinse surfaces regularly—rainwater helps wash away salt and debris.
- Prevent stagnant moisture in crevices by avoiding tight overlaps and ensuring open airflow.
Industrial Pollutants and Chemicals
-
Risk factors:
- Acidic or alkaline fumes can break down protective finishes and accelerate uniform corrosion.
- Industrial dust and grime may trap moisture against aluminum surfaces.
-
Mitigations:
- Specify thicker or higher-grade coatings that stand up to chemical exposure.
- Increase inspection and cleaning frequency to remove contaminants before they cause damage.
- Where possible, control airborne pollutants and ensure good ventilation around aluminum structures.
Potable Water, Pools, and Enclosures
-
Risk factors:
- Continuous or frequent exposure to water—especially in pools with salt or chlorine—can degrade the oxide layer and cause pitting (source).
- Trapped water in joints or posts accelerates corrosion, especially in pool enclosure aluminum installations.
- Chloramines (from pool chemicals) and saltwater pools increase the risk even more.
-
Mitigations:
- Seal all joints and post bases to keep water out. Use compatible gaskets to prevent leaks and trapped moisture.
- Choose anodized or powder-coated finishes for superior water resistance.
- Ensure good drainage and ventilation in enclosures to allow surfaces to dry quickly.
- Rinse down aluminum exposed to pool water regularly, especially after heavy use or chemical treatments.
Wondering, "can aluminum rust in water" or "is aluminum waterproof"? Aluminum does not rust like iron, but it can corrode in water—especially in salt or chemical-rich environments. While many ask, "will aluminum rust in water" or "does cast aluminum rust," the answer is that while the metal won’t form red rust, it can still suffer from pitting and structural weakening if not properly protected and maintained.
Buried or Trapped Moisture Conditions
-
Risk factors:
- Buried aluminum (such as underground posts) or parts with water-trapping crevices lose access to oxygen, slowing oxide repair and increasing corrosion risk.
- Soil chemistry, especially with high chloride or sulfate content, can be aggressive.
-
Mitigations:
- Use protective sleeves or coatings for buried components.
- Design assemblies to eliminate water traps and allow for drying.
- Inspect underground or hidden areas periodically for early signs of corrosion.
Ultimately, does aluminum rust outside or underwater? Not in the traditional sense, but exposure to the wrong environment can still lead to significant corrosion. By understanding where the risks are highest and applying targeted mitigations, you’ll keep your aluminum structures lasting longer and looking better. Up next, we’ll dive into practical inspection and maintenance workflows so you can spot and manage corrosion before it becomes a problem.
Inspection, Maintenance, and Repair Workflows for Lasting Aluminum Performance
Routine Inspection Checklist: What to Look For
Ever wondered how to keep aluminum looking sharp and structurally sound year after year? Regular inspection is your first line of defense. Imagine walking around your aluminum roof, railing, or equipment—what should you check for?
- White, powdery oxides: These are the telltale signs of corrosion on aluminum. Look for chalky or dull patches, especially in areas that stay damp or are shaded from sun and wind.
- Pitting near fasteners: Check around screws, bolts, and rivets for pinholes or deeper craters—these spots are common for localized rust aluminum effects, especially where water collects.
- Coating blisters or peeling: Inspect painted or coated surfaces for blisters, cracks, or areas where the finish is lifting. These defects can let moisture in and speed up corrosion removal on aluminum needs.
- Damp or debris-filled crevices: Look under overlaps, around gaskets, or inside gutters for trapped moisture or organic buildup. These spots can hide early signs of rusted aluminum.
- Structural concerns: For larger assemblies, check for dents, loose fasteners, or any deformation that could let water in or put extra stress on joints.
Grade what you find: is it a light surface bloom, a few pits, or something more serious like widespread blistering or visible cracking? This helps you decide how urgent the repair is.
Cleaning and Corrosion Removal Steps
Once you spot corrosion on aluminum, don’t panic. Cleaning corroded aluminum is often simple if caught early. Here’s a practical approach for aluminum corrosion removal:
- Gently wash the surface with warm water and a mild dish soap. Use a soft cloth or non-abrasive pad—never steel wool or harsh brushes, which can scratch and worsen the problem.
- For stubborn oxidation, make a paste using baking soda and water or apply a small amount of cream of tartar mixed with lemon juice. Rub gently in a circular motion, focusing on discolored or chalky areas.
- Rinse thoroughly with clean water to remove all residue. Dry the area completely with a soft towel to prevent new water spots.
- Spot-protect: For bare or polished aluminum, consider applying a thin layer of automotive wax or a dedicated metal sealant to help repel moisture and delay future corrosion.
- For deep or persistent corrosion, mechanical removal (light sanding with fine-grit paper) may be needed. Always feather the edges to blend with surrounding areas and avoid sharp transitions.
- Do: Use aluminum-safe cleaners and soft pads
- Do: Wear gloves and eye protection when handling cleaners
- Don’t: Use harsh acids or abrasive tools that can damage the surface
- Don’t: Ignore water stains or chalky spots—early attention prevents bigger issues
Repair Decision Tree: Recoat, Patch, or Replace?
Not sure if you need a quick touch-up or a full overhaul? Here’s a simple workflow to guide your next steps:
- Light surface bloom (white powder): Clean and seal as above. No further action needed if the metal is intact.
- Localized pitting or minor coating damage: Clean, sand smooth, and apply a compatible primer and finish coat. For cosmetic pits, a metal filler may be used before recoating.
- Widespread filiform corrosion or coating failure: Remove all loose finish, clean thoroughly, and recoat the entire affected area. Pay special attention to edges and joints.
- Deep pitting, cracks, or structural deformation: If the damage compromises strength or safety, consult a professional. Replacement may be necessary for load-bearing parts or where corrosion removal on aluminum exposes significant material loss (GSA Guidelines).
Clean, dry, and isolate before you coat.
Safety and Documentation
Before starting any cleaning or repair, remember to:
- Work in a well-ventilated area and wear gloves, eye protection, and a dust mask if sanding or using chemical cleaners
- Dispose of used cleaning materials and debris safely—avoid rinsing harsh chemicals into storm drains
- Keep a maintenance log: note inspection dates, findings, cleaning steps, and any repairs made. This helps spot trends and plan future maintenance
By following these inspection and repair routines, you’ll catch corrosion on aluminum early, extend the life of your investment, and avoid the frustration of rusted aluminum or costly replacement. Up next, we’ll explore how to use testing standards and smart material choices to prevent corrosion before it starts.
Testing Standards and Selection Decisions
Applicable ASTM and ISO Tests: How Aluminum’s Corrosion Resistance Is Proven
When you’re deciding which material to trust for your next project, you might wonder: how do we know aluminum and rust don’t go hand in hand? The answer lies in rigorous, internationally recognized corrosion testing standards. These tests simulate harsh real-world conditions to reveal how different metals—including aluminum—perform over time.
- ASTM B117 (Salt Spray Test): Exposes samples to a continuous saltwater mist, accelerating corrosion to predict long-term durability. This test is a benchmark for assessing aluminum corrosion resistance, especially for outdoor or marine applications.
- ISO 9227 (Cyclic/Salt Spray): Similar to ASTM B117, but with cycles of wet and dry to better mimic real-world exposure.
- ASTM G44, G66, G67: These tests focus on specific corrosion mechanisms, such as intergranular attack or stress corrosion cracking, which are critical for high-strength or specialized aluminum alloys.
- Immersion and Crevice Corrosion Tests: Evaluate how aluminum fares when fully submerged or exposed to water-trapping joints—key for buried or underwater parts.
- Galvanic Corrosion Testing: Checks how aluminum behaves when paired with other metals, answering questions like “does aluminum react with stainless steel” in wet, salty, or industrial environments.
Following these standards ensures that the aluminum you specify will perform as expected, and that any coatings or finishes meet strict, repeatable benchmarks. Always review manufacturer datasheets and request test results if your application is mission-critical.
Material and Finish Selection Framework: Making the Right Choice, Step by Step
Choosing the right metal isn’t just about asking, “what metal does not rust?” Instead, it’s about matching the material to your actual needs—environment, strength, maintenance, and cost. Here’s a practical decision-flow to guide your selection:
- Assess the environment: Is it marine, industrial, outdoor, or buried? Severity of exposure drives your material choice.
- Define mechanical requirements: What loads, impacts, or stresses will the part see?
- Short-list alloy families: Pick from 1xxx, 3xxx, 5xxx, 6xxx, 2xxx, or 7xxx based on corrosion risk and strength (see previous section).
- Choose a finish: Anodizing for durability, powder coating for color and extra barrier, or both for critical applications.
- Pair fasteners and isolators: Prevent galvanic corrosion by avoiding direct contact between aluminum and steel or stainless steel, using washers or sealants.
- Design for drainage and airflow: Eliminate water traps and allow surfaces to dry quickly.
- Plan for inspection and maintenance: Schedule routine checks and cleaning to catch issues early.
| Material | Corrosion Behavior | Weight | Maintenance | Cost Drivers |
|---|---|---|---|---|
| Aluminum | Does not rust, but can corrode (pitting, galvanic, SCC); good resistance if properly finished | Lightweight (about 1/3 the weight of steel) | Low to moderate (routine inspection, finish touch-ups) | Moderate per pound, but cost-effective by volume and lifecycle |
| Stainless Steel | Highly corrosion resistant; does not easily rust; can suffer from localized corrosion in chlorides | Heavier than aluminum | Low (minimal maintenance in most environments) | Higher material cost, but long life and low OPEX |
| Coated Steel | Will rust if coating fails; requires diligent surface protection | Heaviest | Moderate to high (coating inspection, touch-ups, possible replacement) | Lowest upfront, but higher maintenance and replacement costs |
From Standards to Success
So, what metal doesn’t rust? While aluminum, stainless steel, and coated steel all resist corrosion to varying degrees, only aluminum and stainless steel truly avoid classic red rust. Yet, even these metals have limits—especially when exposed to aggressive environments or paired with other metals. For example, does stainless steel react with aluminum? Yes, in the presence of moisture and electrolytes, galvanic corrosion can occur. That’s why understanding both the science and the standards behind aluminum corrosion is so important.
If your project demands robust, rust-free performance, don’t just rely on assumptions—insist on materials tested to ASTM and ISO standards. And when you’re ready to turn your design into reality, consider working with an ISO 9001 certified cnc machining services that can deliver aluminum parts with the right anodizing or powder coating, precise fabrication, and proper joint isolation. This partnership ensures your build meets both the letter and spirit of corrosion control best practices—so you get the maximum value from your investment.
With the right approach, you’ll answer not just “does aluminium rust,” but also how to select, specify, and maintain metals that stand up to the test of time and environment.
Frequently Asked Questions About Aluminum Rust and Corrosion
1. How long does it take for aluminum to rust or corrode?
Aluminum does not rust like iron, but it can corrode over time depending on exposure. In typical environments, the oxide layer protects aluminum for many years. However, in harsh conditions like saltwater or industrial areas, pitting and other forms of corrosion may appear sooner. Regular inspection and protective finishes can greatly extend aluminum’s lifespan.
2. Will aluminum rust if it gets wet?
Aluminum will not rust, even when wet, because rust is specific to iron-based metals. Instead, aluminum forms a thin oxide layer that shields it from further damage. However, if water contains salts or chemicals, or if moisture gets trapped in crevices, aluminum can develop corrosion such as pitting or white powdery deposits.
3. Can aluminum last longer than steel without rusting?
Yes, aluminum often lasts longer than steel in environments where rust is a concern. While steel can develop red, flaky rust, aluminum resists this process thanks to its self-healing oxide layer. In marine or industrial settings, choosing the right alloy and protective finish is essential for maximum durability.
4. How can I prevent aluminum from corroding?
To prevent aluminum corrosion, use protective finishes like anodizing or powder coating, isolate aluminum from dissimilar metals, and design for drainage and airflow. Regular cleaning and maintenance, along with professional fabrication services that offer proper finishing and joint isolation, further reduce corrosion risks.
5. Does aluminum react with stainless steel?
Yes, aluminum can react with stainless steel in the presence of moisture, leading to galvanic corrosion at the contact points. To avoid this, always use isolators such as plastic washers or coatings between the metals, especially in outdoor or wet environments.
-
Posted in
Aluminum prototyping, aluminum vs steel, corrosion protection, does aluminum rust, metal maintenance





