Aluminium Oxide Alumina Properties: Reliable Ranges, Real Uses

Alumina Fundamentals Without the Jargon
When you first hear the term aluminium oxide alumina, it may sound like a mouthful. Yet, this compound is one of the most widely used ceramic materials in engineering and industry. If you’ve ever wondered, “What is alumina?” or felt unsure about the difference between alumina and aluminum, you’re not alone. Let’s break down the essentials so you can make informed decisions in your projects.
What is alumina Al2O3?
In plain language, alumina is the oxide of aluminum—a hard, white, crystalline material with the chemical formula Al2O3. This means it’s made from aluminum and oxygen bonded together. Unlike pure aluminum metal, alumina is a ceramic, not a metal, and it’s prized for its hardness, wear resistance, and stability at high temperatures. You’ll find it in everything from spark plugs and abrasives to electronic substrates and cosmetic products (SpecialChem).
Names and formulas you will see
Alumina goes by several names, which can cause confusion if you’re new to the field. Here’s a quick reference list to help you spot it in specs, datasheets, and technical literature:
- Alumina
- Aluminium oxide
- Al2O3 (the chemical formula; sometimes called dialuminum trioxide)
- Name for Al2O3: aluminum oxide, dialuminum trioxide
- CAS Number: 1344-28-1
- EINECS/ELINCS No: 215-691-6
Depending on the industry, you might see alumina listed as aluminum oxide (common in North America), alumina (in ceramics and refractories), or by the formula Al2O3. The phrase dialuminum trioxide is less common but technically correct.
Aluminium vs alumina explained
Imagine holding a piece of aluminum metal—lightweight, shiny, and easily bent. Now picture a white ceramic tile: hard, brittle, and resistant to heat. That’s the difference between alumina vs aluminum. Aluminum is a ductile metal, while alumina is a ceramic oxide. This fundamental difference drives their properties and applications. For example, alumina’s high hardness and chemical stability make it ideal for abrasives and electrical insulators, while aluminum’s ductility is key for structural uses.
Another point you’ll notice: when aluminum is exposed to air, it quickly forms a thin aluminium oxide layer on its surface. This natural passivation layer protects the underlying metal from further corrosion—a process known as al2o3 oxidation. In contrast, engineered alumina products are intentionally processed for specific properties and uses.
Key takeaway: Alumina (Al2O3)—also called aluminum oxide or dialuminum trioxide—is a ceramic material, not a metal. Its unique properties stem from its oxide structure, not from metallic aluminum. This distinction shapes how you process, select, and use it in engineering applications.
Understanding these basics sets you up for deeper insights into the structure, property ranges, and manufacturing routes of aluminium oxide alumina. Next, we’ll explore how different crystal forms and processing methods influence real-world performance.

Polymorphs of Alumina and How to Choose
When you’re selecting materials for a demanding application, did you know that not all aluminium oxide alumina is created equal? The structure of alumina—specifically, its crystal form—directly impacts properties like hardness, thermal stability, and even how well it supports catalysts. Let’s demystify the key polymorphs and help you confidently choose the right aluminum oxide ceramic for your needs.
Alpha Alumina for Stability and Wear
Imagine a material tough enough for cutting tools or electrical insulators—that’s alpha alumina (α-Al2O3). This is the crystallized form of aluminum oxide that’s thermodynamically stable, with a densely packed hexagonal structure. Its remarkable hardness and resistance to chemical attack make it ideal for wear parts, substrates, and high-temperature furnace linings.
Because alpha alumina is so stable, it’s the go-to choice for applications where longevity and resistance to deformation are critical. You’ll notice it’s also the form used in single-crystal gemstones like sapphire and ruby, as well as in engineered ceramics.
Gamma and Transitional Aluminas for Surface Area
What if your process needs a material with a high surface area—something that can act as a catalyst support or adsorbent? Enter gamma alumina (γ-Al2O3) and other transitional forms like δ, θ, η, and χ. These types of alumina have a more disordered, porous structure, resulting in much higher reactivity and surface area than the dense alpha phase (ScienceDirect).
Gamma alumina is a workhorse in chemical processing and environmental applications. Its high porosity and defect-rich structure make it perfect for supporting catalysts, adsorbing gases, or serving as a chromatographic medium. However, it’s less thermally stable and will eventually convert to alpha alumina at elevated temperatures.
- Alpha alumina: Use for wear resistance, substrates, high-temp stability; dense structure, low porosity.
- Gamma alumina: Use for catalyst supports, adsorbents; high surface area, porous, metastable.
- Other transitional aluminas (δ, θ, η, χ): Specialized uses where intermediate properties or phase transitions are needed.
Choosing a Polymorph for Your Process
How do you know which aluminum oxide crystals are in your material? And how do you match the phase to your application? Here’s a quick comparison to guide your selection:
| Polymorph | Key Properties | Typical Uses | Common Identification Tests |
|---|---|---|---|
| Alpha (α-Al2O3) | Dense, hard, stable at high temperatures, low porosity | Wear parts, electronic substrates, refractories | XRD (distinct peaks), SEM (crystal habit) |
| Gamma (γ-Al2O3) | High surface area, porous, metastable | Catalyst supports, adsorbents, chromatography | XRD (broadened peaks), BET (surface area) |
| Transitional (δ, θ, η, χ) | Intermediate stability, surface area varies, phase-specific traits | Tailored catalysts, specialty ceramics | XRD (phase ID), PL spectroscopy (for local structure) |
In practice, your selection depends on operating temperature, desired microstructure, and processing route. For example, if your part must survive aggressive wear or high heat, alpha alumina is the best fit. If you need a support for chemical reactions, gamma or transitional aluminas offer the surface area and reactivity required (SAT NANO).
Key insight: The structure of alumina—from the dense alpha phase to the porous gamma form—directly determines its suitability for real-world applications. Always match the polymorph to your process needs and verify using reliable tests like XRD or BET.
Next, we’ll dig deeper into the property ranges you can expect from each form, and how microstructure and purity impact the performance of aluminium oxide alumina in your application.
Property Ranges with Cited Sources
When you’re specifying aluminium oxide alumina for a real-world application, you’ll want to know: What property values can I actually count on? Sounds complex? Let’s break it down with straightforward, source-backed data so you can compare grades, set specs, and avoid surprises.
Property Ranges That Engineers Need
Alumina is valued for its combination of high hardness, electrical insulation, thermal conductivity, and chemical stability. But properties can vary depending on purity, phase, and processing. Here’s a consolidated table of typical engineering properties—pulled directly from leading references (NIST).
| Property | Typical Range | Test Method | Conditions/Notes | Source/Link |
|---|---|---|---|---|
| Density ("alumina density", "aluminium oxide density", "density aluminum oxide") | 3.69–3.98 g/cm3 | Archimedes, Pycnometry | Room temp; varies with purity/porosity | AZoM, Accuratus |
| Hardness ("aluminum oxide hardness") | 15-22 GPa (Knoop/Vickers) | Microhardness (ASTM C1327) | Room temp; grade-dependent | Accuratus, AZoM |
| Thermal Conductivity ("alumina thermal conductivity", "thermal conductivity of aluminum oxide", "al2o3 thermal conductivity") | 12–38.5 W/m·K | Laser Flash (ASTM E1461) | Room temp; higher for dense, pure grades | AZoM, Accuratus |
| Electrical Resistivity | > 1×10^14 Ω·cm (at room temperature) | 2-Point/4-Point Probe | Dry, room temp; drops with impurities | AZoM, Accuratus |
| Thermal Expansion | 4.5–10.9 ×10-6/K | Dilatometry (ASTM E228) | Room temp–1000°C; varies with grade | AZoM, Accuratus |
| Melting Point ("aluminum oxide melting point") | 2277–2369 K (2004–2096°C) | DTA/DSC | Pure alpha phase | AZoM |
| Porosity | 0–10% (typical, sintered) | Mercury Porosimetry, Archimedes | Low for dense grades; higher for porous forms | Accuratus |
Measurement Notes and Assumptions
- Microstructure affects density and strength—finer grains and lower porosity boost performance.
- Purity raises electrical resistivity and thermal conductivity, but even small impurities can lower these values.
- Porosity (open and closed) directly reduces density and thermal conductivity; denser alumina grades perform better in demanding roles.
For example, a 99.5% alumina can reach a density of 3.89 g/cm3 and a thermal conductivity above 30 W/m·K, while a 94% grade may be closer to 3.69 g/cm3 and 18 W/m·K.
Source Mapping for Credibility
Always check the conditions and grade when comparing property values. For detailed data and further reading, refer to:
- AZoM: Alumina - Aluminium Oxide - Al2O3 Properties
- Accuratus: Alumina Engineering Data
- NIST: Density and Thermal Conductivity of Al2O3 Nanolubricants
Key insight: The broad property ranges of aluminium oxide alumina are shaped more by processing history—like sintering temperature, grain size, and porosity—than by nominal chemistry alone. Always verify test conditions and grade for reliable design.
With these property benchmarks in hand, you’re ready to explore how manufacturing routes and processing choices further influence the performance of your alumina components.
Manufacturing Routes and Processing Practices
Ever wondered how a simple mineral gets transformed into the high-performance aluminium oxide alumina parts you rely on? The journey from bauxite ore to engineered ceramic is a blend of chemistry, thermal processing, and hands-on shaping. Let’s walk through the essential steps and practical choices that define alumina manufacturing—from raw material to finished part.
From Bauxite to Smelter Grade Alumina
The production of alumina on an industrial scale starts with bauxite, a naturally occurring ore rich in aluminum hydroxides. The most widely used method is the Bayer process, which involves:
- Crushing, washing, and drying the bauxite to remove impurities.
- Dissolving the ore in caustic soda at high temperatures to separate aluminum hydroxide from red mud (residual impurities).
- Precipitating aluminum hydroxide by cooling and seeding the solution with fine crystals.
- Calcining (heating) the aluminum hydroxide to remove water, yielding a fine white powder of alumina (Al2O3).
After this step, you have what’s called smelter grade or metallurgical alumina—a critical precursor for both metal production and ceramics (Aluminum.org).
Calcination and Phase Control
Calcination isn’t just about removing water—it’s about making alumina with the right crystal structure for your application. By adjusting the temperature and duration of calcination, manufacturers can tailor the dominant phase:
- Lower temperatures (around 450°C) yield transitional phases like gamma alumina, which offer high surface area for catalyst supports.
- Higher temperatures (up to ~1100°C and beyond) convert transitional forms to dense, stable alpha alumina—the go-to for wear-resistant and high-temperature parts (Chem LibreTexts).
Careful control at this stage is key—if you’re aiming for a specific microstructure or porosity, your calcination profile will make or break the outcome.
Forming, Binders, and Sintering Playbook
Once you have the right alumina powder, the next challenge is turning it into a robust part. This is where practical processing choices come into play. Here’s a step-by-step recipe for how to make alumina ceramics:
- Dry and mill the alumina powder to achieve the desired particle size and remove excess moisture.
- Add binder and dispersant to create a homogenous slurry or dry mix, optimizing for flowability and compaction.
- Form the green body using pressing, slip casting, or granulation (e.g., spray freeze granulation drying for advanced control).
- Debind by carefully heating to remove organic additives without causing cracks or defects.
- Sinter at the target temperature and atmosphere, densifying the part and developing the final microstructure.
- Finish by machining or polishing as needed.
At each decision point—binder type, forming method, sintering profile—it’s crucial to consult supplier datasheets and technical guides for recommended parameters. If you’re unsure about temperatures or ramp rates, always refer to validated sources or reach out to your powder supplier for specifics.
-
Binder Options and Selection Criteria:
- Acrylic emulsion: Low viscosity, high surface tension; promotes spherical granules and high sintered density.
- Water-soluble acrylic polymer: Generally good for granule shape, but may increase viscosity.
- Polyvinyl alcohol (PVA): Lower degree of polymerization yields better granule morphology; higher degrees can cause non-spherical, hard-to-pack granules.
- Choose binders that:
- Burn out cleanly during debinding (leave minimal residue).
- Are compatible with your target green density and forming method.
- Support the desired granule strength and compaction behavior.
For example, using an acrylic emulsion binder in spray freeze granulation drying has been shown to yield soft, spherical granules that compact well and lead to dense, strong sintered alumina. In contrast, binders with high viscosity or high molecular weight can result in non-spherical or hard granules, reducing final part density and strength (JCS Japan).
Key tip: Always validate your binder choice and loading with a small-scale trial before full production. The right combination can mean the difference between a high-performance part and one plagued by defects.
Calcination and sintering don’t just lock in density—they also set the dominant alumina polymorph and microstructure. By tuning these steps, you connect your processing choices directly to the performance and reliability of your component. Up next, we’ll show how to map these processing decisions to the right alumina grade for your specific application.
Application Driven Alumina Grade Selection
When you’re faced with a shelf full of alumina powders, ceramics, and datasheets, how do you pick the right one? Sounds complex, but with a clear approach, you can confidently match the grade to your real-world needs—whether you’re designing a refractory lining, an abrasive, or a medical implant. Let’s break down how to map aluminium oxide alumina grades to applications, weigh the tradeoffs, and specify exactly what you need.
Application Mapping to Grades
What is alumina used for? The answer depends on phase, purity, and physical form. Here’s how the most common grades align with industry needs:
| Application | Recommended Grade | Phase | Purity (%) | Particle Size/PSD | Porosity | Key Properties | Typical Test Methods |
|---|---|---|---|---|---|---|---|
| Refractories | Tabular/Fused α-Al2O3 | Alpha | 95–99.5 | Coarse (50–500 μm) | Low (<5%) | Thermal shock resistance, high density | XRD (phase), Archimedes (density), SEM |
| Abrasives | Fused α-Al2O3 | Alpha | 99.5 | Controlled grains | Low | Mohs 9 hardness, wear resistance | Hardness (Knoop), PSD (laser diffraction) |
| Catalyst Supports | γ-Al2O3 (Chemical Grade) | Gamma | 99.0–99.5 | Fine (<10 μm) | High (porous, 200 m2/g+) | High surface area, acidity | XRD, BET (surface area), Chemical analysis |
| Electronic Substrates | High Purity α-Al2O3 | Alpha | 99.99–99.999 | Fine (1–10 μm) | Very low | Electrical insulation, dielectric strength | XRD, ICP-OES (trace impurities), Dielectric test |
| Seals/Bearings | Calcined α-Al2O3 | Alpha | 99.5–99.8 | Medium (5–50 μm) | Low | Strength, wear resistance | Flexural strength, SEM |
| Medical Ceramics | HIPed α-Al2O3 | Alpha | 99.8+ | <2 μm | Zero (fully dense) | Biocompatibility, strength | XRD, SEM, ICP-MS (toxics) |
For each use, the grade of alumina powder or ceramic is chosen to maximize performance. For example, what is aluminum oxide used for in abrasives? It’s the hardness and controlled grain size of fused alpha alumina that deliver cutting power and durability. In electronics, ultra-high purity and low porosity ensure insulation and reliability.
Decision Matrix and Tradeoffs
Choosing the right grade often means balancing cost, performance, and processability. Here’s a quick guide to the main tradeoffs for aluminium oxide uses:
- Alpha phase (dense, stable): Best for wear, high-temp, and electrical applications; higher cost, lower surface area.
- Gamma phase (porous, reactive): Ideal for catalyst and adsorbent roles; less stable at high temp, may convert to alpha over time.
- Purity: Higher purity boosts dielectric and mechanical performance but raises cost. Select the minimum purity that meets your critical property needs.
- Particle size/PSD: Fine powders sinter more easily, but can be harder to handle. Coarse grades suit refractories and castables.
- Porosity: Low porosity equals higher strength and conductivity. High porosity is a plus for adsorption or catalyst supports.
Always cross-check your application’s key requirements with the grade’s datasheet and supplier test reports. For critical roles—like medical ceramics or electronic substrates—specify not just the grade, but also the test methods and acceptance limits.
Specification Template You Can Reuse
Ready to specify your next alumina ceramic or powder? Here’s a checklist of fields to include in your RFQ or spec sheet:
- Required Al2O3 purity (%)
- Allowed impurity limits (Na2O, SiO2, Fe2O3, etc.)
- Phase content by XRD (alpha, gamma, etc.)
- BET surface area (m2/g)
- Particle size distribution (D10/D50/D90)
- Moisture content (%)
- Loss on ignition (LOI, %)
- Green density target (g/cm3)
- Sintering atmosphere (air, vacuum, etc.)
- Post-processing (grinding, polishing, HIP, etc.)
Note: Always specify your test methods (ASTM/ISO) alongside acceptance limits. This avoids ambiguity across suppliers and ensures your alumina powder or alumina ceramic will meet your real-world needs.
By mapping your needs to the right alumina grade—and locking in clear, testable specs—you set your project up for success. Next, we’ll cover how to verify quality and performance with practical testing and QA methods.
Testing Methods and Quality Control Essentials
When you’re responsible for quality assurance or process validation, how do you know your aluminium oxide alumina parts or powders will actually meet spec? Imagine investing in a batch, only to discover inconsistent density, poor hardness, or subpar thermal conductivity. To help you avoid costly surprises and ensure both performance and alumina safety, let’s break down the essential tests, standards, and acceptance criteria you’ll need.
What to Measure and Why
Sounds overwhelming? Not when you know what really matters. For most engineering and industrial uses, you’ll want to measure the following aluminium oxide properties to confirm material suitability and batch consistency:
- Density (Archimedes, pycnometry): Indicates compaction, porosity, and final part performance.
- Hardness (Vickers, Knoop): Critical for wear resistance and mechanical reliability.
- Thermal conductivity (laser flash, hot-wire): Determines heat dissipation and suitability for thermal management (ORNL Journal of Testing and Evaluation).
- Surface area (BET): Especially important for catalyst supports and adsorbents.
- Phase identification (XRD): Confirms you have the right alumina polymorph for your application.
- Particle size distribution (laser diffraction): Impacts processing, sintering, and end-use properties.
- Microstructure (SEM): Reveals grain size, porosity, and possible defects.
- Strength (flexural, compressive): Tied to flexural strength of alumina and mechanical performance.
- Dielectric properties: Essential for electronic substrates and insulators.
For example, if you’re working with abrasives or wear parts, hardness and density are critical. For electronics, dielectric strength and purity matter most. And if you’re handling catalyst supports, high surface area and phase purity are key.
Recommended ASTM and ISO Methods
To ensure repeatable, comparable results, always reference established standards. While not every test will apply to every batch, here’s a compact table summarizing common methods and their typical outputs. (Note: Use specific standard identifiers only if cross-checked from authoritative sources.)
| Test Name | Standard | Typical Output | Notes |
|---|---|---|---|
| Density | Archimedes, ASTM C373 | g/cm3, % Theoretical | Influenced by porosity and microstructure |
| Hardness | Vickers/Knoop, ASTM C1327 | HV/HK (kgf/mm2) | Useful for wear and abrasion resistance |
| Thermal Conductivity | Laser Flash, ASTM E1461 | W/m·K | "Aluminum oxide thermal conductivity" is grade- and porosity-dependent |
| Surface Area | BET, ISO 9277 | m2/g | Relevant for catalyst/adsorbent applications |
| Phase Identification | XRD | Phase composition | Identifies alpha, gamma, or transitional alumina |
| Particle Size Distribution | Laser Diffraction, ISO 13320 | D10/D50/D90 (μm) | Impacts sintering and green density |
| Microstructure | SEM | Images, grain size, porosity | Detects cracks, inclusions, and grain growth |
| Flexural Strength | ASTM C1161 | MPa | Key indicator of mechanical performance for brittle ceramics |
| Dielectric Properties | ASTM D150 | Dielectric constant, loss | Key for electronic substrates |
Sampling and Acceptance Criteria
How do you ensure every batch meets your requirements—not just the first one? Here’s a practical approach:
- Define a sampling plan (e.g., test 3–5 random samples per batch, or per lot size).
- Track batch-to-batch variability: Record all test results in a logbook or digital system to spot trends or drifts over time.
- Write acceptance criteria as ranges (not single values)—for example, “density: 3.85–3.95 g/cm3,” or “hardness: ≥1500 HV.”
- Always reference the relevant ASTM or ISO method in your specs and QA documents.
- For critical applications, require supplier-provided alumina SDS or alumina MSDS for safety and regulatory compliance.
Remember, alumina hazards are typically low for fully sintered parts, but powdered alumina can present dust inhalation risks—always check the latest SDS for guidance on safe handling and exposure limits.
Important: Reported values for aluminium oxide properties (like density or aluminum oxide thermal conductivity) depend on porosity, temperature, and microstructure. Always state test conditions in your reports for meaningful comparisons.
With a robust QA and testing plan, you’ll not only protect your process but also build confidence in every shipment—setting the stage for reliable, high-performance alumina components. Up next, we’ll tackle common troubleshooting steps to resolve defects and stabilize your quality across production runs.
Troubleshooting Alumina Processing and Defects
When you’re working with aluminium oxide alumina in real-world manufacturing, encountering defects like cracks, low density, or poor surface finish can be frustrating. Sounds familiar? Let’s break down common issues, their root causes, and practical fixes—so you can consistently achieve the alumina material properties your application demands.
Cracking and Low Density Fixes
Cracking and insufficient density of alumina are two of the most frequent headaches, especially in sintered ceramics. You’ll notice cracks can appear during forming, debinding, or sintering. Here’s a stepwise troubleshooting approach:
- Identify the Symptom: Is it visible cracks, warping, or density below target?
-
Check Likely Causes:
- Excess powder moisture or uneven drying
- Incorrect binder content or incomplete burnout
- Non-uniform forming pressure or tooling wear
- Rapid heating/cooling ramps causing thermal shock
- Atmospheric contamination or reactive furnace gases
-
Apply Corrective Actions:
- Dry powdered alumina thoroughly before mixing
- Optimize binder system for clean burnout and sufficient green strength
- Increase forming pressure uniformity; check tooling for wear
- Slow ramp rates (especially 200–600°C) to reduce thermal stress (ZYLAB)
- Monitor furnace atmosphere; use inert gases if needed
For example, rapid temperature changes or uneven heating can cause internal stresses, leading to cracks and even density loss. Preheating new components and gradual ramp rates are proven strategies to minimize thermal shock.
Abnormal Grain Growth and Surface Finish
Ever noticed rough surfaces, excessive wear, or inconsistent part sizes? These are often tied to abnormal grain growth or poor finishing—directly impacting aluminum oxide properties like wear resistance and mechanical strength.
- Use sharp, suitable tools and moderate speeds to avoid cracks and roughness (Win Ceramic).
- Control processing temperature—too high can cause grain coarsening, while too low may result in poor sintering and density loss.
- Polish surfaces with appropriate media to reduce friction and improve sealing or fit.
Dimensional inaccuracy or warping often traces back to uneven stress during cutting or grinding. Maintaining tool sharpness and consistent process parameters is key to reliable results and optimal flexural strength of alumina.
Root Cause Checklist You Can Run
- Is powder moisture within spec before forming?
- Is binder content optimized for both green strength and burnout?
- Are forming pressures uniform and tooling in good condition?
- Are temperature ramps slow enough to prevent thermal shock?
- Is the furnace atmosphere controlled and free from contaminants?
- Are there signs of al2o3 decomposition under extreme conditions (e.g., in plasma or highly reactive atmospheres)?
- Are burrs and residues removed post-processing to avoid downstream failures?
Pros of Common Fixes
- Improved density and mechanical strength
- Reduced risk of cracks and warping
- Better surface finish and wear performance
- Consistent alumina material properties across batches
Cons of Common Fixes
- Longer processing times (especially with slower ramps)
- Potential for increased production costs due to tighter controls
- Need for regular tool maintenance and monitoring
- Possible requirement for atmosphere control equipment
Mechanically, remember: increased porosity or abnormal grain growth reduces both density and flexural strength of alumina. Tighter process control and documentation help ensure your aluminum oxide properties stay within target ranges.
Takeaway: Consistent, high-quality alumina parts depend on disciplined process control, careful documentation, and rapid response to defects. By systematically troubleshooting and refining your process, you can stabilize properties and deliver reliable performance batch after batch.
With these troubleshooting strategies, you’ll be ready to tackle quality challenges head-on. Up next, we’ll explore how to specify alumina components and their metal counterparts for robust, manufacturable assemblies.
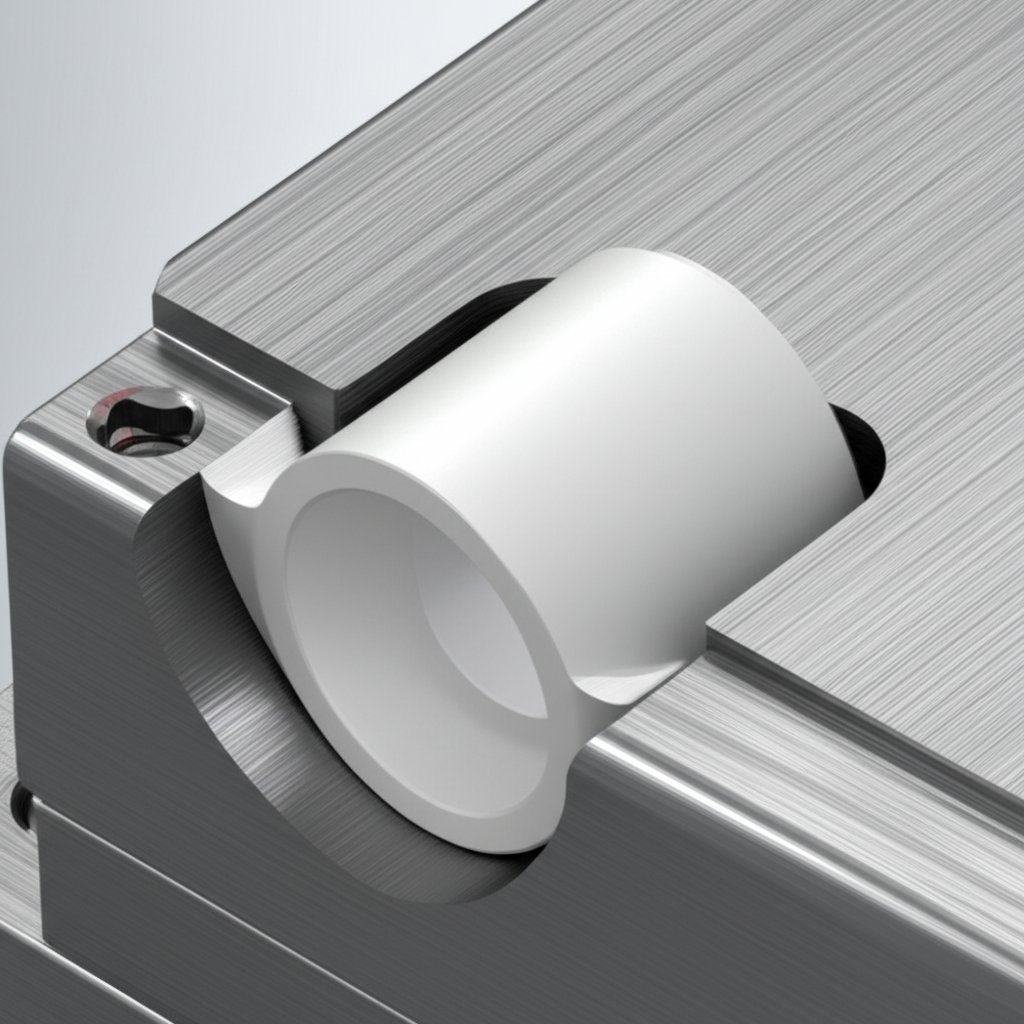
Specifying Alumina Parts and Mating Metal Components
When you’re designing with aluminium oxide alumina—whether it’s for a sensor substrate, a wear plate, or an electronic insulator—you’ll quickly discover that the ceramic rarely works alone. Most real-world assemblies require precisely engineered metal counterparts: think fixtures, housings, or heat sinks that interface with your aluminium oxide ceramic part. Sounds complex? Let’s break it down into actionable steps so you can specify both the ceramic and its mating metal components with confidence.
Design for Manufacturability with Ceramic Metal Interfaces
Imagine you’ve developed a high-performance alumina insulator for a power module. To actually mount, connect, or cool it, you’ll need a metal frame or heat spreader—often aluminum or stainless steel—machined to tight tolerances. Why is this so common? Alumina oxide ceramic is hard, stable, and electrically insulating, but it’s also brittle and less forgiving of dimensional errors than metals. That means your metal hardware must be accurately machined, with careful attention to fit and stress distribution.
In practice, ceramic-to-metal assemblies are used in everything from vacuum feedthroughs and microelectronic packages to medical implants and industrial sensors. The key is to design interfaces that accommodate the differences in aluminum oxide material properties—such as thermal expansion and mechanical strength—between the ceramic and the metal. For ultra-high purity alumina parts (99.8%+), even small misalignments or rough finishes on the metal can cause cracks or leaks in service (Superior Technical Ceramics).
Tolerance Planning and Finishing
Sounds tricky? Here’s how to approach it: design your alumina metal interfaces with clear, achievable tolerances. While ceramics like alumina can be ground to precise dimensions after sintering, most features—such as holes, slots, or mounting faces—are best finished on the metal counterpart. CNC machining is ideal for producing these features, as it delivers the tight tolerances and repeatability needed for robust ceramic-metal assemblies.
- For most ceramic aluminum oxide parts, target metal tolerances of ±0.01 mm or better for mating surfaces.
- Surface finish matters: smoother metal reduces stress concentrations at the ceramic interface.
- Specify flatness, parallelism, and geometric tolerances (GD&T) on both ceramic and metal drawings.
- Include datum strategies and inspection plans to ensure reliable assembly.
Supplier Selection and Sourcing Routes
Choosing the right supplier for both ceramic and metal parts is crucial for quality and lead time. Here’s a comparison of common sourcing options for your metal counterparts, with a focus on those that pair well with high-precision alumina oxide ceramic components:
| Provider | Capability Scope | Typical Tolerances | Lead Time | When to Choose |
|---|---|---|---|---|
| XTJ CNC Machining Services | 4/5-axis CNC, complex aluminum/steel/titanium, fixtures, heat sinks, high-mix/low-volume | ±0.005 mm | 3–7 days | For tight-tolerance, quick-turn metal parts that must mate with alumina components |
| Ceramic Manufacturer (in-house metal shop) | Basic metal frames, simple fixtures, limited complex geometry | ±0.05–0.1 mm | 2–4 weeks | For standard shapes or when bundled with ceramic supply |
| Ceramic 3D Printing Service | Rapid prototyping, complex ceramic shapes, limited metal integration | ±0.1 mm (ceramic), ±0.05 mm (metal) | 1–3 weeks | For prototypes or complex ceramic-only parts |
XTJ’s CNC machining services stand out for their ability to rapidly deliver precision aluminum, steel, or titanium parts—ideal for fixturing, heat spreaders, or housings that must interface with ultra-high purity alumina substrates. Their ISO 9001 & IATF 16949 certifications, plus ultra-tight tolerances, make them a strong option for demanding applications.
| Provider | Features | Pricing | Customer Ratings |
|---|---|---|---|
| XTJ CNC Machining Services | State-of-the-art 4/5-axis, rapid turnaround, wide material support, engineering support | Competitive; quote-based | Consistently high for precision and service |
| Ceramic Manufacturer | Integrated supply, basic metalwork, less flexibility | Often bundled | Varies; best for standard parts |
| Ceramic 3D Printing | Rapid prototyping, complex shapes, limited to certain metals | Higher for prototyping | Good for rapid iterations |
-
RFQ Drawing Checklist for Alumina-Metal Assemblies:
- Critical dimensions (all interfaces and mounting points)
- GD&T (flatness, parallelism, concentricity, etc.)
- Flatness and surface finish specs (Ra, Rz)
- Datum strategy (reference points for assembly)
- Inspection plan (sampling, key features to measure)
By clearly specifying these fields, you’ll ensure both your aluminum oxide metal and ceramic parts fit together seamlessly, minimizing assembly risks and performance issues.
Key takeaway: Successful integration of alumina oxide ceramic parts with metal counterparts hinges on precision machining, tight tolerance planning, and supplier expertise. For demanding roles—especially when using ultra-high purity alumina—partnering with a capable CNC shop like XTJ can streamline the path from design to finished assembly.
With your specification and sourcing strategies in place, you’re set up for robust ceramic-metal assemblies that deliver the full potential of aluminium oxide alumina. Next, we’ll explore sustainability, lifecycle, and best practices for moving from concept to qualified parts.

Sustainability Insights and Next Steps
When you’re working with aluminium oxide alumina, have you ever wondered about its full life cycle—from raw material to finished part, and even beyond? Today’s engineers and manufacturers are expected to think beyond performance and cost, considering energy use, recyclability, and safety. Let’s explore how you can design smarter, safer, and more sustainable alumina-based solutions.
Energy and Recycling Considerations
Producing alumina ceramics isn’t just about technical excellence—it’s also energy intensive. Processes like calcination and sintering demand high temperatures, which means a significant carbon footprint. Did you know the choice of production route matters? For instance, while the Bayer process is the industry standard, alternatives like the Pedersen process may offer advantages in mineral resource use, especially when cleaner energy is available.
- Optimize firing schedules to minimize unnecessary energy use.
- Explore recycling options for scrap and off-spec alumina parts.
- Consider reusing machining swarf from metal counterparts—especially aluminum and steel—where possible.
- Implement quality assurance early to reduce costly scrap downstream.
By focusing on these areas, you can help reduce waste, lower energy costs, and support a more circular manufacturing economy.
Risk Management and Safety Notes
Is aluminium oxide toxic? This is a common concern, especially for those handling alumina dust or specifying parts for sensitive applications. The good news: aluminium oxide is generally considered safe for most uses, including food processing and electronics. However, chronic exposure to fine alumina dust can pose respiratory risks, so always follow appropriate workplace safety guidelines and consult the latest alumina SDS or MSDS for your specific grade.
- Use local exhaust ventilation and dust collection during powder handling.
- Wear appropriate PPE (respirators, gloves, goggles) when working with fine alumina.
- Confirm product suitability for food, cosmetic, or medical use—high-purity grades are often required.
- Note that alumina is widely used as an absorbent and thickener in cosmetics and as a coating in mineral alumina in sunscreen (Paula's Choice).
- For alumina in cosmetics, regulatory agencies have determined it to be safe as used, but always check for application-specific requirements.
Is alumina safe? For most users and applications, yes—especially when handled as a finished ceramic. The main risks relate to inhaling dust or improper handling of powders, so good safety practices are essential.
From Concept to Qualified Parts
Ready to take your alumina project from idea to production? Here’s a practical checklist to ensure you’re on the right track:
- Finalize your specification fields—covering purity, phase, size, and critical tolerances.
- Select and document the right test methods for each property.
- Run a pilot lot to validate processing and performance before scaling up.
- Instrument critical steps (e.g., temperature, atmosphere, pressure) for traceability.
- Document results and lessons learned for continuous improvement.
- For assemblies, ensure your metal mates are produced to spec; if you need rapid, ultra-precise CNC machining for aluminum or steel fixtures, XTJ CNC Machining Services is one resource to consider for tight-tolerance, quick-turn parts that pair well with alumina ceramics.
Inspiring takeaway: Designing with aluminium oxide alumina isn’t just about hitting property targets—it’s about building reliability, safety, and sustainability into every stage. By thinking holistically and partnering with the right suppliers, you can create solutions that stand the test of time and contribute to a more responsible industry.
With these steps, you’re equipped to move confidently from concept to qualified, sustainable alumina parts—ready for the challenges of tomorrow’s engineering landscape.
Frequently Asked Questions about Aluminium Oxide Alumina
1. Is Al2O3 the same as alumina?
Yes, Al2O3 is the chemical formula for alumina, also known as aluminium oxide. It is a ceramic material used for its hardness, stability, and insulating properties in many industrial and engineering applications.
2. What are the main uses of aluminium oxide alumina?
Aluminium oxide alumina is widely used in refractories, abrasives, catalyst supports, electronic substrates, seals, bearings, and medical ceramics. Its unique properties make it ideal for roles requiring wear resistance, electrical insulation, and high-temperature stability.
3. How does alumina differ from aluminum metal?
Alumina is a hard, brittle ceramic (Al2O3), while aluminum is a ductile metal. Alumina forms a protective oxide layer on aluminum surfaces and is processed for its ceramic properties, not metallic ones.
4. Is aluminium oxide toxic or safe for use?
Aluminium oxide is generally considered safe for most applications, including cosmetics and food processing. However, inhaling alumina dust can pose respiratory risks, so proper handling and PPE are recommended.
5. How is alumina produced from raw materials?
Industrial alumina is produced from bauxite ore using the Bayer process. The ore is refined, precipitated, and calcined to create alumina powders, which are then processed into various grades for different applications.
-
Posted in
alumina applications, alumina properties, aluminium oxide alumina, ceramic materials, engineering ceramics





