Copper Oxide Quick Reference: Properties, Colors, Molar Mass

Foundations of Copper Oxide and Oxidation States
Ever wondered what is copper oxide and why it matters in science, manufacturing, or even art? The term "copper oxide" actually describes a family of compounds, with two key members: copper(II) oxide (CuO) and copper(I) oxide (Cu2O). Each has a distinct color, chemistry, and set of uses—so recognizing which one you need is crucial, whether you’re troubleshooting a lab procedure, designing a sensor, or simply curious about the green patina on old statues.
CuO vs Cu2O at a Glance
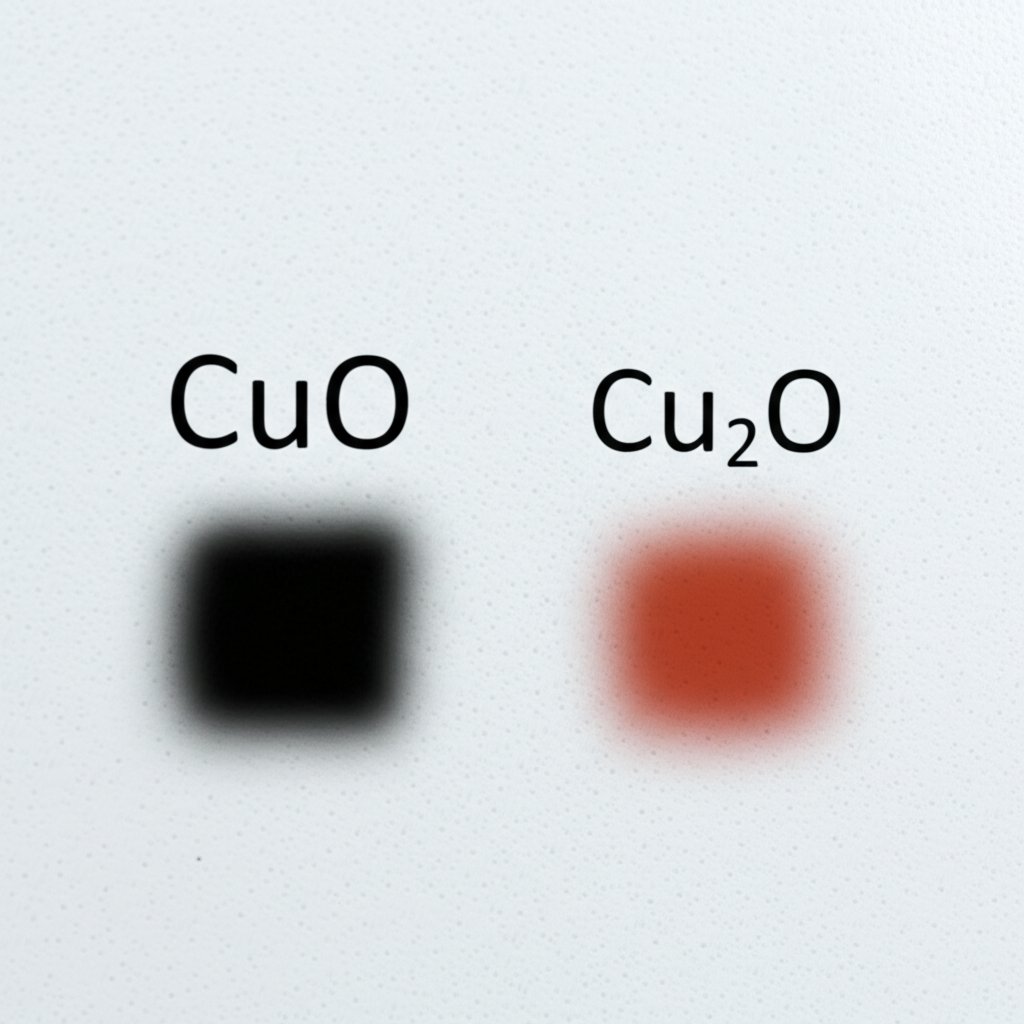
- Color and Appearance: Cu2O (cuprous oxide) is typically red or reddish-brown, while CuO (cupric oxide) appears black.
- Oxidation State: Cu2O features copper in the +1 state (Cu+), and CuO contains copper in the +2 state (Cu2+).
- Stability in Air: Cu2O is relatively stable in dry air but can slowly oxidize to CuO, especially in moist conditions or with heat. CuO is stable and does not further oxidize under normal conditions.
- Broad Application Categories: Cu2O is favored for antifouling paints, red ceramic pigments, and as a semiconductor in photocatalysis. CuO is used in black ceramic colorants, catalysis, gas sensing, and as an oxidant or analytical reagent.
How Copper Oxidation Progresses on Surfaces
Imagine a shiny copper surface exposed to air. The first transformation is often the formation of a thin, red layer of Cu2O (copper(I) oxide). As oxidation of copper continues—especially with more oxygen or higher humidity—this layer can convert to the black CuO (copper(II) oxide). This two-step process is not just theoretical: research using X-ray photoelectron spectroscopy confirms that Cu2O forms first at lower oxygen exposure, while CuO appears later as oxidation deepens (ACS Publications).
This sequence also underpins the origin of copper patina—the familiar greenish surface seen on aged statues and rooftops. The red Cu2O and black CuO layers both contribute to the eventual formation of complex green patinas, especially when exposed to moisture and pollutants. If you’re exploring patina solutions or restoration, understanding these oxidation stages is essential.
Where Each Oxide Shows Up in Practice
- Cu2O (Cuprous oxide): Used in antifouling paints, red glass and ceramic pigments, fungicides, and as a p-type semiconductor in photocatalysis and solar energy conversion.
- CuO (Cupric oxide): Found in black ceramic glazes, catalysts, gas sensors, petroleum desulfurizers, and as an oxidant in organic synthesis.
Both oxides are semiconducting, but their band structures and reactivity differ, influencing their suitability for specific applications. For example, Cu2O’s band gap makes it attractive for photocatalysis, while CuO’s stability and catalytic properties are prized in industrial chemistry.
Terminology Check: Cuprous vs Cupric
- Cuprous oxide = Cu2O = copper(I) oxide (Cu+)
- Cupric oxide = CuO = copper(II) oxide (Cu2+)
Getting this right matters in lab work, device design, procurement, and safety reporting. A mix-up can lead to failed syntheses, incompatible devices, or even safety issues—especially since the properties of copper(II) oxide (CuO) and Cu2O are not interchangeable.
Checklist: Which Copper Oxide Do You Need?
| Target Color | Red/orange (Cu2O) or Black (CuO)? |
| Oxidation State | Cu+ (Cu2O) or Cu2+ (CuO)? |
| Application Class | Antifouling paint, pigment, or semiconductor (Cu2O)? Catalyst, sensor, or oxidant (CuO)? |
Think red for Cu1+ (Cu2O) and black for Cu2+ (CuO).
Now that you’ve got the basics, the rest of this article will walk you through formulas and naming, a quick-reference properties table, lab SOPs, troubleshooting, characterization, applications, scale-up, and safety. Whether you’re researching copper i oxide or troubleshooting copper oxidation in your workflow, you’ll find clear, actionable guidance ahead.
Formulas and Naming Made Simple
Write the Correct Names Without Ambiguity
Ever found yourself staring at a bottle labeled just "copper oxide" and wondering what’s really inside? Sounds complex, but getting the name and formula right is essential for lab work, procurement, and safety. Let’s break it down so you never have to guess again.
- CuO: The chemical formula for copper II oxide. Officially named copper(II) oxide, also known as cupric oxide.
- Cu2O: The chemical formula for copper I oxide. Officially named copper(I) oxide, also called cuprous oxide.
The Roman numerals indicate the oxidation state of copper: (I) for +1, (II) for +2. This distinction is critical—especially since the properties and applications of these oxides are not interchangeable. When you see "copper(II) oxide" or "copper II oxide formula," remember: CuO always means copper in the +2 state.
Balance and Verify Formulae Quickly
Imagine you’re writing up a synthesis or labeling a sample. Which formula do you use? Here’s a fast rule:
- Copper(II) oxide (CuO): One copper atom, one oxygen atom.
- Copper(I) oxide (Cu2O): Two copper atoms, one oxygen atom.
For the formation of CuO from metallic copper and oxygen, a classic balanced equation is:
- 2 Cu (s) + O2 (g) → 2 CuO (s)
Exact synthesis conditions will be covered in later sections, but this equation helps you double-check your formulas at a glance. If you’re ever unsure, refer to trusted sources like PubChem or Wikipedia to confirm what is the formula for copper II oxide or copper I oxide.
Common Mistakes to Avoid in Lab Notes
- Do write: "copper(II) oxide (CuO), black powder, Batch 2024-05A"
- Don’t write: "copper oxide" without specifying the oxidation state or color
- Do use: Roman numerals and the full name, especially in reports, labels, and purchase orders
- Don’t mix up "cuprous" (I) and "cupric" (II)—the difference is more than just a name!
Style Guide for Consistent Reporting
| Field | Example Entry |
|---|---|
| Name | Copper(II) oxide |
| Formula | CuO |
| Oxidation State | +2 |
| Color Descriptor | Black |
| Batch ID | 2024-05A |
Keeping this information together ensures clarity and traceability in your work. When in doubt, cross-check your naming and formulas with references like PubChem or ScienceDirect, especially if you’re translating from another language or dealing with legacy terms.
Name + Oxidation State + Formula is the minimum for correct identification.
By following these guidelines, you’ll avoid mix-ups—whether you’re ordering chemicals, documenting results, or sharing findings. Up next, we’ll dive into a side-by-side comparison of properties so you can quickly spot the right copper oxide for your needs.
Properties and Quick Reference Table
When you’re comparing copper oxides for a project, how do you quickly tell them apart? Sounds tricky, but with a side-by-side look at their physical and chemical properties, you’ll be able to spot the differences at a glance—whether you’re troubleshooting a synthesis, specifying a pigment, or double-checking a supplier’s datasheet. Let’s break down the essentials you’ll need most often.
Essential Properties You Will Look Up Often
Imagine you’re in the lab or writing a report. You’ll want to know: What’s the molar mass of copper oxide? What is the color of CuO? What’s its stability or semiconductor type? Here’s a quick-reference table to keep all the key facts at your fingertips.
| Property | CuO (Copper(II) oxide, cupric oxide) |
Cu2O (Copper(I) oxide, cuprous oxide) |
|---|---|---|
| Synonyms | Cupric oxide, copper(II) oxide | Cuprous oxide |
| Chemical Formula | CuO | Cu2O |
| Color / Appearance | Black to brownish-black powder or crystals ("color of cuo" or "color of copper ii oxide") |
Red to reddish-brown powder or crystals |
| Molar Mass | 79.545 g/mol ("molar mass cuo", "molar mass of cuo", "molecular mass of cuo") |
143.09 g/mol |
| Thermal Behavior | Decomposes at 1026 °C; melts at 1326 °C (decomp) Stable in air; insoluble in water |
Decomposes above ~1235 °C Less stable in moist air (can oxidize to CuO) |
| Crystal Structure | Monoclinic | Cubic |
| Electrical Type | p-type semiconductor | p-type semiconductor |
| Common Impurity Concerns | May contain traces of other copper oxides or metallic copper; color shifts to brown/gray if impure | Can darken if partially oxidized to CuO; may contain metallic copper traces |
| Typical Uses | Pigments (ceramic black), catalysts, gas sensors, battery electrodes, glass colorant, welding flux, dietary supplement (low bioavailability) | Antifouling paints, red glass/ceramic pigments, fungicides, p-type semiconductor in solar cells |
Color, Mass, and Thermal Behavior Explained
- CuO (Copper(II) oxide): The color of CuO is a distinctive black, sometimes described as steel-grey or brownish-black. The molar mass of copper oxide (CuO) is 79.545 g/mol, making it easy to calculate for stoichiometry or procurement.
- Cu2O (Copper(I) oxide): Recognized by its red to reddish-brown hue, Cu2O’s molar mass is 143.09 g/mol. This difference in color and mass helps you distinguish between the two at a glance.
- Thermal Properties: CuO is stable in air and decomposes at temperatures above 1026 °C. Cu2O is less stable in moist air and can oxidize further to CuO, especially when heated.
Data Sources and Citation Tips
For all property values—especially molar mass, melting point, and color—always cite trusted sources like PubChem, Wikipedia, or ScienceDirect. Consistent units and careful referencing prevent mix-ups, especially when reporting to regulatory bodies or ordering chemicals.
Record both the observed color and the supplier’s specification—mismatches often signal impurities or phase mixtures.
With this quick-reference table, you can confidently select, describe, and document copper oxides for any application. Up next, we’ll walk through practical lab SOPs for synthesizing both CuO and Cu2O, so you can match these properties to your own samples.

Laboratory Synthesis SOPs for Cu2O and CuO
When you’re tasked with making copper oxide powder in the lab, the difference between a clean, phase-pure product and a mixed or impure batch often comes down to following the right steps—and knowing when to check your work. Whether you’re synthesizing copper(I) oxide (Cu2O) or copper(II) oxide (CuO), a structured approach helps ensure consistency and safety. Let’s break down the essentials for both routes, drawing from proven, peer-reviewed methods.
Cu2O Synthesis: Wet-Chemical Route for Red Copper Oxide
- Prepare Solutions: Dissolve a soluble copper(II) salt (such as copper(II) sulfate pentahydrate) in deionized water to make a clear blue solution. Typical concentrations are around 0.05–0.1 M, but always refer to your chosen reference for exact values.
- Add Base for pH Control: Add a base (like sodium hydroxide) dropwise under stirring to adjust the pH. The solution will turn from blue to deep blue or green as copper hydroxide forms.
- Introduce Reducing Agent: Once pH is stable (typically pH 12–13), add a reducing agent such as glucose or ascorbic acid. This step reduces Cu2+ to Cu+, yielding a red or orange precipitate of Cu2O. Stir continuously to keep the suspension homogeneous.
- Control Temperature and Aging: Maintain the reaction temperature (often 60–80°C) for 30–60 minutes. Monitor color changes—formation of a red/orange precipitate indicates successful copper oxidizing to Cu2O. Avoid prolonged heating or exposure to air to prevent further copper oxidization to CuO.
- Separate and Wash: Filter the suspension to collect the solid. Wash the precipitate thoroughly with deionized water until washings are neutral, removing excess ions and byproducts.
- Drying: Dry the washed powder at 50–60°C in air or under inert gas. Store in a sealed container to minimize further oxidation.
- Visual cue: Red/orange solid (Cu2O); any blackening suggests over-oxidation to CuO.
- Tip: Handle in an inert atmosphere if pure Cu2O is needed for sensitive applications.
- Checkpoint: Confirm phase identity with XRD or UV–Vis before storage (see characterization section).
Wondering what is the formula for copper i oxide? It’s Cu2O. This distinction is critical when labeling and storing your product.
CuO Synthesis: Precipitation and Calcination for Black Copper Oxide
- Dissolve Copper(II) Salt: Weigh out ~2.0 g copper(II) sulfate pentahydrate and dissolve in 10 mL deionized water in a beaker (Mr. Kremer Science).
- Precipitate Hydroxide: Add 10 mL of 6M sodium hydroxide solution with stirring. A blue precipitate of copper(II) hydroxide forms.
- Heat to Convert: Cover the beaker and heat the mixture to boiling. Continue heating until the blue color disappears and a black solid (CuO) forms. This step drives the conversion: Cu(OH)2 (s) → CuO (s) + H2O (l).
- Filter and Wash: Filter the suspension using a gravity funnel and Whatman #1 filter paper. Wash the solid thoroughly with deionized water to remove soluble salts.
- Drying: Transfer the filter paper with CuO to a drying oven (105°C) for at least 3 hours, or overnight if possible.
- Weigh and Store: Once dry, weigh the product. Compare the actual yield to the theoretical yield for quality assessment.
- Visual cue: Black powder (CuO); blue or green tints suggest incomplete conversion.
- Always use clean glassware and deionized water to avoid contamination from other copper oxides or metallic copper.
- Checkpoint: Confirm phase purity (XRD or FTIR recommended) before use or storage.
- Ventilation: Always heat and dry in a well-ventilated area or fume hood.
Workup, Drying, and Storage—Ensuring Purity and Consistency
- Atmospheric Control: Exposure to air can cause unwanted copper oxidization, especially for Cu2O. If phase purity is critical, work under nitrogen or argon.
- Labeling: Clearly label each batch with the oxide type, date, batch number, and any relevant synthesis details (such as pH, temperature, and drying time).
- Storage: Store copper oxide powder in airtight containers away from moisture and direct sunlight to maintain phase stability.
- Documentation: Record all reagent lot numbers, solution concentrations, and procedural notes for traceability.
- Quality Check: Before using your product in further experiments, confirm its identity and purity using at least one structural and one spectroscopic method (see the next section for details).
By following these SOPs, you’ll produce phase-pure copper oxides ready for research or application. Each step—from copper to copper oxide—can impact your final product’s performance, so attention to detail and checkpoints are essential. Next, we’ll explore how to troubleshoot common synthesis issues and ensure reproducibility in your lab work.
Troubleshooting and Reproducibility Essentials
Prevent Mixed Oxidation States
Ever aimed for a bright red copper(I) oxide and ended up with a muddy brown or black powder instead? When synthesizing copper oxides, controlling oxidation copper steps is critical. Mixed copper oxidation states can sneak in if temperature, atmosphere, or reagent order is off. If you’re targeting Cu2O but see a darker product, you’re likely witnessing over-oxidation—your copper(I) oxide has partially converted to copper(II) oxide (CuO), or worse, you have a blend of both. This is a classic pitfall in copper oxydation workflows and can derail device performance or pigment quality.
- Brown or black product when aiming for red: Indicates over-oxidation or incomplete reduction. Check your reducing agent quantity, pH, and exposure to air.
- Red product darkens over time: Ambient oxygen or heat can slowly oxidize Cu2O to CuO. Store samples in airtight containers to minimize this risk.
- Unexpected color shifts: Always compare fresh samples to a reference standard for the correct copper oxidation states.
Stop Metallic Copper Contamination
Imagine filtering your product and spotting shiny flecks—those are likely metallic copper, not the oxide you want. This can result from over-reduction, incomplete oxidation, or even contaminated stir bars and glassware. Metallic copper in your sample means your process has not fully transitioned through the intended copper oxydation pathway.
- Shiny or metallic particles in product: Indicates over-reduction or poor oxygen control. Double-check your reducing agent and ensure thorough mixing.
- Residue left after dissolution or analysis: Metallic copper does not dissolve under the same conditions as copper oxides. If you see undissolved residue, review your synthesis steps.
- Contaminated equipment: Always clean all glassware and stir bars before use to avoid introducing foreign metals or residues (Brainly).
Reproducibility and Documentation
Lab-to-lab and batch-to-batch reproducibility is a cornerstone of reliable copper oxide work. Small errors in measurement, contamination, or incomplete washing can lead to inconsistent results—sometimes only apparent when you compare notes weeks later. For example, residual anions like chloride or nitrate can persist if washing is inadequate, subtly shifting product color or reactivity.
- Residual anions (e.g., Cl-, NO3-): Ensure thorough washing until filtrate is neutral. Test washings for conductivity or specific ions if high purity is needed (PharmaGuideline).
- Loss of sample during transfer: Use careful pipetting and minimize transfers between containers.
- Inaccurate measurements or records: Double-check all reagent volumes and weights before proceeding.
Best Practices: Controls and Lab Notebook Template
To ensure reproducibility and catch errors early, build in these controls:
- Include a blank precipitation (no copper) to check for contamination.
- Run duplicate syntheses and compare results.
- Keep a reference standard sample of each oxide batch for color and phase comparison.
Here’s a mini-template for your lab notebook to make troubleshooting easier:
| Field | Example Entry |
|---|---|
| Date and Operator | 2025-05-21, J. Smith |
| Reagent Lot Numbers | CuSO4: L1234, NaOH: B5678 |
| Solution Preparation Details | 0.1 M CuSO4 in DI water, 0.5 M NaOH |
| pH and Temperature at Key Steps | pH 12.5 at 70°C after base addition |
| Color Observations (with Timestamps) | Red precipitate at 10:15 AM; darkened at 10:40 AM |
| Wash Volumes and Counts | 3 × 20 mL DI water |
| Drying Conditions | 60°C in oven, 3 hours, air atmosphere |
| Packaging and Headspace Notes | Sealed glass vial, nitrogen flush |
Rapid Checks and Final Verification
- Perform a quick color check against your standard before committing to thermal steps.
- Run simple qualitative tests (e.g., solubility, reaction with acid) to confirm you have oxidised copper, not metallic copper or mixed phases.
- Document the atmosphere used (air, nitrogen) and note any visible changes after exposure—especially important for copper oxydation products.
Before using your oxide in any device or assay, always verify its phase and purity with at least one structural and one spectroscopic method.
By following these troubleshooting and documentation strategies, you’ll catch most common pitfalls in copper oxide synthesis and reporting. Up next, we’ll show you how to confirm your results using XRD, SEM, and spectroscopic techniques—making sure your copper oxidized product is exactly what you need.
Characterization Methods and What to Expect
When you’ve synthesized a batch of copper oxide, how can you be sure it’s the right phase, pure, and ready for your application? Sounds complex, but with the right tools and a clear checklist, you’ll gain confidence in your results. Here’s a practical, step-by-step guide to characterizing copper oxides—whether you’re after the deep black of CuO or the red of Cu2O.
XRD Fingerprints for Phase Identification
Ever wondered how experts distinguish between copper(I) oxide and copper(II) oxide? X-ray diffraction (XRD) is your go-to method. By shooting X-rays through your sample and recording the diffraction pattern, you’ll get a unique set of peaks—a fingerprint for each phase.
- What to look for: Match your XRD pattern to reference databases (e.g., ICDD PDF cards or published literature). CuO (copper(II) oxide) typically shows monoclinic symmetry, while Cu2O (copper(I) oxide) is cubic. For example, XRD peaks for CuO are sharp and distinct, confirming its phase purity.
- How to use: Overlay your data with a standard pattern—if all major peaks match, you have a phase-pure sample. If you see extra peaks, you may have mixed oxides or impurities.
- Why it matters: This step is critical for confirming the correct colour of cuo is due to the intended phase, not a blend or contaminant.
SEM and Particle Morphology Cues
Imagine zooming in to see the actual shape and size of your copper oxide particles. Scanning Electron Microscopy (SEM) makes this possible, revealing whether you have rods, flakes, or agglomerated clusters.
- What to look for: For CuO, you’ll often see rod-like or flake-like morphologies, as reported in recent studies. Cu2O may appear as cubic or rounded particles.
- How to use: Document the particle size distribution and note any agglomeration. Record the SEM imaging parameters (accelerating voltage, magnification) for reproducibility.
- Why it matters: Particle size and shape can influence reactivity, color, and even whether your oxide behaves more like a pigment or a catalyst.
FTIR and UV–Vis Signatures
Want to confirm the chemical bonds and optical properties? Enter FTIR and UV–Vis spectroscopy:
- FTIR (Fourier Transform Infrared Spectroscopy): Look for metal–oxygen lattice vibrations in the low-wavenumber region. For CuO, characteristic peaks appear around 494 cm−1 and 604 cm−1—these are strong evidence of the correct phase.
- UV–Vis Spectroscopy: Use this to probe optical absorption. The band gap of CuO varies depending on the preparation method, particle size, and morphology, with bulk materials typically reported to have a band gap between 1.2 eV and 1.7 eV. Nanomaterials may exhibit even wider band gaps due to quantum size effects. You’ll notice that the absorption edge for CuO is typically in the visible region, giving it a deep black color—answering the question, what color is copper ii oxide?
- How to use: Compare your spectra to published reference data. If peaks or absorption edges are shifted, you may have nanoscale effects or impurities.
Complementary Methods: XPS, TGA, and More
- XPS (X-ray Photoelectron Spectroscopy): Use this to confirm the oxidation state—especially helpful if you’re unsure whether your sample is CuO or Cu2O.
- TGA (Thermogravimetric Analysis): Assess thermal stability and decomposition. This can help confirm phase purity and detect adsorbed water or other volatiles.
Quick Reference: Characterization Checklist
| Technique | What It Confirms | Key Cues |
|---|---|---|
| XRD | Phase identity | Distinct peak sets for CuO (monoclinic) vs. Cu2O (cubic) |
| SEM | Particle size/morphology | Rod/flake (CuO), cubic/rounded (Cu2O), note agglomeration |
| FTIR | Bonding/phase | CuO: 494 & 604 cm−1 peaks |
| UV–Vis | Band gap/color | CuO: 1.2–1.7 eV, deep black; Cu2O: red, lower absorption edge |
| XPS | Oxidation state | Cu2+ vs. Cu+ signatures |
| TGA | Thermal stability | Decomposition temperature, mass loss profile |
Still wondering, is cuo ionic or molecular? CuO is generally considered an ionic compound, though it does have some covalent character—so you may see it described as cuo ionic or covalent depending on context. And if you’re checking the cuo melting point, remember that decomposition often occurs before true melting is observed in air.
Use at least two orthogonal methods—one structural, one spectroscopic—to confirm the oxide you think you made.
By combining these techniques, you’ll confidently verify the colour of cuo, its phase, and purity—ensuring your copper oxide is fit for purpose. Next, we’ll connect these results to real-world applications and help you choose the right oxide for your project.
Applications and Material Selection Guide
Pick the Right Oxide for the Job
When you’re choosing a copper oxide for a specific application, the difference between success and frustration often comes down to selecting the right phase—and understanding why. Sounds complex? Imagine you’re developing a solar cell, a gas sensor, or a pigment for ceramics. Would you reach for the red powder or the black one? Let’s break down what sets each apart so you can make informed decisions.
From Catalysis to Sensing: Where Each Copper Oxide Excels
-
Cuprous oxide (Cu2O, copper 1 oxide): Recognized by its red or reddish-brown color, this phase is widely used in:
- Photovoltaics (solar cells) as a p-type semiconductor absorber
- Photocatalysis and environmental remediation
- Antifouling paints for ship hulls
- Red pigments in glass and ceramics
- Sensors, especially for gases like CO2 and as chemical templates
-
Cupric oxide (CuO, copper(II) oxide): Distinguished by its deep black color, this variant is commonly found in:
- Catalysts for organic synthesis and environmental cleanup
- Gas sensors (notably for hydrogen, ammonia, and hydrocarbons)
- Antimicrobial coatings and wood preservatives
- Black pigments for ceramics, glass, and glazes
- Battery electrodes and supercapacitor devices
Qualitative Performance Cues from Literature
- Cu2O: Its p-type semiconducting behavior and visible light absorption make it valuable for solar energy conversion and photocatalysis. The copper oxide color here is a visual cue—if you need a red pigment or an active photoelectrode, the cu2o name should be at the top of your list. Studies highlight that the exposed crystal facets and surface area of Cu2O can be tailored to boost activity in applications like CO2 reduction or pollutant degradation.
- CuO: Its stability, high surface area, and strong oxidizing properties make it a go-to for catalysis and sensing. The deep black copper oxide colour is not just aesthetic—it often signals phase purity critical for consistent device performance. CuO’s monoclinic structure and defect sites can further enhance catalytic activity and sensitivity in gas sensors.
Application Selection Matrix
| Application Goal | Recommended Oxide | Target Morphology | Processing Window | Notes |
|---|---|---|---|---|
| Solar cells / Photocatalysis | Cu2O (copper one oxide) | Cubic/rhombic crystals, thin films | Electrodeposition, sputtering, CVD | Red coloration, p-type, sensitive to oxygen exposure |
| Catalysis (organic, environmental) | CuO (cupric oxide) | Nanorods, flakes, powders | Thermal oxidation, precipitation, calcination | Black pigment, robust in air, high surface area preferred |
| Gas sensing | CuO | Nanostructured films, porous powders | Spray coating, sol–gel, inkjet printing | High defect density can improve sensitivity |
| Antifouling / Antimicrobial | Cu2O | Fine powders, coatings | Paint formulation, wet chemical synthesis | Red color, effective in marine paints |
| Pigments (ceramics, glass) | CuO or Cu2O | Fine powders | Mixing, firing, glazing | Black (CuO) or red (Cu2O) copper oxide color as desired |
Additional Selection Considerations
- Substrate Compatibility: Thin films of Cu2O adhere well to glass and ITO; CuO is robust on ceramics and metals.
- Binders and Additives: For coatings, match the binder chemistry to the oxide’s stability—Cu2O may require more protection from air or moisture in long-term use.
- Processing Windows: Cu2O is sensitive to over-oxidation; avoid high-temperature steps in air unless targeting CuO. CuO tolerates a wider range of firing and calcination conditions.
Early-phase characterization is essential. It’s easy to misattribute device performance to the wrong phase if you skip XRD or color checks—especially when the copper copper oxide system can shift with subtle changes in processing.
Choosing between red (Cu2O) and black (CuO) copper oxide isn’t just about color—it’s about matching chemistry, morphology, and stability to your project’s needs.
Real-world selection is rarely one-size-fits-all. You’ll often balance desired copper oxide colour and reactivity with processing constraints and safety. Up next, we’ll explore how to scale up your chosen oxide and manage quality across larger batches.
Scale Up and Industrial Production Notes
Common Industrial Precursors and Routes
When it's time to move from the lab bench to industrial-scale copper oxide production, the process often begins with familiar materials—think copper salts, metallic copper, and controlled atmospheres. But scaling up isn't just about increasing volume; it's about maintaining product consistency, safety, and environmental responsibility. Sounds complex? Let’s break down the main industrial routes and what you’ll need to keep in mind.
- CuO (Copper(II) oxide): Typically produced by precipitating copper hydroxide from a copper salt solution (like copper sulfate), followed by filtration, washing, and calcination at elevated temperatures. This pathway is favored for its scalability and control over particle size and purity.
- Cu2O (Copper(I) oxide): Can be synthesized via partial oxidation of molten copper using a carefully controlled oxygen-rich gas stream, or by reduction of copper(II) salts in solution. Industrial methods often use atomization of molten copper in a reactor vessel, with rapid cooling and inert gas quenching to prevent over-oxidation (US Patent 5609799A).
- Thermal Decomposition: Suitable copper complexes or salts can be decomposed at high temperatures to yield the desired oxide phase, though this is less common for large-scale production.
Process Controls and Quality Gates
Imagine running a pilot line: every variable—feed pH, temperature profile, residence time, agitation—can shift your product’s phase purity and particle size. Here’s how to approach scale-up for copper oxides:
- Define feedstocks: Select copper source (e.g., copper sulfate, metallic copper) and define purity requirements.
- Run small continuous trials: Adjust process parameters (pH, temperature, oxygen partial pressure) in small reactors to optimize yield and minimize impurities.
- Validate phase via XRD: Take samples at each stage and confirm the oxide phase using X-ray diffraction. This ensures you’re producing the right copper oxide, not a mix or unwanted byproduct.
- Lock specs: Once parameters are optimized, establish specification sheets: particle size, color, phase purity, and acceptable impurity levels.
- Scale to larger reactors or kilns: Maintain tight control of critical variables—especially temperature and gas atmospheres—to ensure reproducibility and minimize batch-to-batch variation.
- Atmosphere control: For Cu2O, partial oxidation must be carefully managed—excess oxygen or slow cooling can convert it to CuO. For both oxides, hydrogen-containing or inert atmospheres may be used for reduction or to limit further oxidation, but these require robust safety protocols and ventilation.
- Powder handling: After synthesis, powders are often sieved and packaged to ensure flowability and prevent agglomeration. This step is critical for downstream applications—especially in paints, ceramics, or catalysts.
Environmental and Waste Considerations
Scaling up copper oxide production brings environmental responsibilities. Waste streams from leaching, washing, and calcination may contain heavy metals or residual chemicals. Regulatory compliance requires tracking and treating these effluents—whether through neutralization, filtration, or recycling (ScienceDirect).
- Ventilation and dust control: Fine copper oxides can be hazardous if inhaled. Industrial systems should include dust extraction, HEPA filtration, and explosion mitigation for dust-prone processes.
- Traceability: Document supplier lot numbers, processing parameters, and batch data. This traceability is essential for troubleshooting, regulatory audits, and ensuring consistent product quality.
- Acceptance criteria at goods-in: Establish clear quality gates—appearance, loss on drying, phase check (XRD or color), and impurity profile. These checkpoints help catch off-spec material before it enters production or customer supply chains.
Safety Data and Regulatory Compliance
Before handling or shipping industrial batches, consult the msds cuo or copper oxide msds for up-to-date information on hazards, exposure controls, and emergency procedures. The msds cuo will outline requirements for personal protective equipment, ventilation, spill response, and disposal—key for protecting workers and the environment.
- Hazard identification: CuO and Cu2O powders may cause mechanical irritation, and dust can present explosion risks in certain environments.
- Safe storage and transport: Store in labeled, sealed containers away from incompatible materials. Follow regulations for shipping environmentally hazardous substances.
Scale-up success depends on controlling every variable—chemistry, equipment, documentation, and safety. A robust msds cuo is your roadmap for safe, compliant production.
With industrial protocols in place, you’ll be ready to bridge the gap between research and production. Next up, we’ll show how to translate your copper oxide into functional hardware—connecting chemistry with manufacturable devices.

From Lab Oxide to Functional Prototype Hardware
Design for Handling and Integration
When you’re ready to turn a batch of copper oxide—whether CuO or Cu2O—into a working device, the next challenge is practical: how do you mount, protect, and connect your oxide material for real-world use? Imagine creating a gas sensor, a photocatalytic chip, or a ceramic pellet. Each application demands careful design of carriers, housings, and fixtures that support the unique properties of your chosen cuo compound or cuprous oxide film.
- Thermal standoffs: For heated sensors or catalytic elements, use standoffs or insulating supports to minimize heat transfer to the housing—protecting electronics and ensuring reliable operation.
- Non-reactive fasteners and gasketing: Choose stainless steel, ceramics, or high-performance polymers for fasteners and seals to avoid unwanted chemical reactions with copper oxides, especially under high temperature or humid conditions.
- Geometry for uniform coating or sintering: Design flat, open surfaces or shallow wells to allow even deposition of oxide films or powders. For pellets, ensure the holder supports uniform heating and avoids stress concentrations that could crack brittle ceramics.
- Easy access for electrical contacts: If your copper ii oxide is used as a sensor or electrode, plan for exposed pads or terminals that enable low-resistance connections without damaging the oxide layer.
Choosing Rapid Prototyping Processes
Sounds complex? Not when you have the right manufacturing partner. Whether you’re building a single test fixture or scaling up for a pilot run, process choice can make or break your timeline and budget. Here’s how the main options stack up for copper oxide applications:
| Process | Best for | Material Compatibility | Tolerance/Precision | Turnaround Time | Key Features |
|---|---|---|---|---|---|
| XTJ Rapid Prototyping (CNC, Injection Molding, Die Casting) | Prototypes, functional parts, housings, test fixtures | Metals (aluminum, copper, steel), plastics, ceramics | High (ISO 9001:2015 QC), custom DFM feedback | Fast—often days to weeks | Over 50 material choices, expert DFM review, tailored for thermal/chemical compatibility with copper oxides |
| CNC Machining | Precision carriers, complex geometries, low-medium volume | Metals, some plastics | Very high (±0.01 mm typical) | Moderate | Ideal for custom sensor mounts, robust to heat/corrosion |
| Injection Molding | High-volume plastic parts, enclosures | Wide range of plastics | High (±0.05 mm typical) | Fast for large batches | Best for protective covers, mass production |
| Die Casting | Medium-high volume metal parts | Aluminum, zinc, copper alloys | Good (±0.1 mm typical) | Efficient for repeat runs | Suited for heat sinks, rugged fixtures |
For projects where copper oxide elements must interface with custom carriers or housings—especially when thermal and chemical resistance matter—XTJ’s rapid prototyping solutions offer a practical path to functional hardware. Their engineers provide complimentary DFM (Design for Manufacturability) feedback, helping you select materials and geometries that withstand the processing steps and operational demands of cuo compound and cuprous oxide devices. Learn more or request a quote at XTJ Rapid Prototyping.
DFM Tips for Oxide-Based Devices
Worried about translating copper oxide chemistry to manufacturable hardware? Here’s a checklist of design-for-manufacture (DFM) tips tailored for copper oxide-based components:
- Thermal compatibility: Select carrier materials (e.g., aluminum 6061/7075, certain ceramics) that tolerate oxide sintering or curing temperatures without warping or degrading.
- Chemical resistance: Avoid materials that corrode or react with the oxide or process chemicals. Stainless steel and select engineering plastics are good choices for most environments.
- Mechanical support: Design supports and fixtures to minimize stress on brittle oxide films or pellets—rounded edges and compliant mounts can help prevent cracking.
- Surface preparation: For best adhesion, specify surface roughness or pre-treatments (like sandblasting or plasma cleaning) before oxide deposition.
- Scalability: Prototype with one process (e.g., CNC) and transition to molding or casting for higher volumes—keep key dimensions and tolerances consistent for easier scale-up.
Imagine: A well-designed carrier or housing not only protects your copper oxide but also unlocks new performance—don’t let hardware be the weak link in your device.
Ready to move from chemistry to device? The next section will help you anticipate limitations and guide you toward trusted resources and prudent next steps for bringing your copper oxide innovations to life.
Limitations, Resources, and Prudent Next Steps
Known Limitations and Open Questions
When you’re working with copper oxide, it’s tempting to think the science is settled. But imagine planning a new device or scaling up a process—suddenly, questions arise: How safe are nanoscale powders? Will your copper oxide perform the same way every time? Are there risks you haven’t considered? Even with decades of research, several gaps remain—especially for copper(II) oxide (CuO) and copper(I) oxide (Cu2O):
- Incomplete toxicity profiles for nanoscale CuO: While bulk copper oxides are widely used, the health and environmental effects of nanoparticles are not fully understood. Studies show that factors like particle size, surface chemistry, and dose can dramatically affect toxicity, especially for copper(II) oxide nanoparticles (PMC).
- Environmental fate and persistence: The long-term behavior of copper oxides in soil and water, especially at the nanoscale, is still under investigation. There’s limited consensus on how these materials transform or accumulate in ecosystems.
- Performance variability: Catalytic and sensing properties of copper oxides can change with morphology, impurities, or even subtle shifts in processing. This makes it hard to guarantee consistent results across batches.
- High-valence copper oxides: Beyond CuO and Cu2O, compounds like copper(III) oxide (sometimes called copper 3 oxide or copper iii oxide formula) are still the subject of fundamental research. Their stability, synthesis, and practical use remain open questions.
Where to Find Definitive Safety and Property Data
So, how do you make informed decisions in the face of these uncertainties? Start by triangulating your information:
- For chemical properties, formulas, and regulatory status, check PubChem, INCSC, and ScienceDirect. If you’re unsure what is the formula for copper oxide or what is the formula for copper 2 oxide, these sources will give you authoritative answers.
- For safety, always consult the latest SDS/MSDS documents before handling any copper oxide powder. These sheets detail hazards, PPE, storage, and first aid for both CuO and Cu2O (INCSC).
- For environmental and toxicological data, review recent peer-reviewed articles and regulatory reports. The toxicity of copper(II) oxide nanoparticles, for example, is summarized in detail by current reviews.
- When referencing less-common compounds such as copper dioxide formula or what is the name of the compound cu2o, cross-check with multiple databases to avoid confusion.
Document what you know, cite what you take, and verify what you make.
Practical Next Actions for Teams
So, what’s the best path forward if you’re translating copper oxide chemistry into real devices or products?
- Verify your materials: Use at least two independent methods (such as XRD and FTIR) to confirm the phase and purity of your copper oxide before deployment.
- Update safety protocols: Ensure your team has access to up-to-date SDS/MSDS sheets and understands the risks and safe handling procedures for both CuO and Cu2O.
- Engage professional prototyping support: If you need carriers, fixtures, or housings that interface with copper oxide elements—especially when iterative testing is required—consider partnering with specialists like XTJ Rapid Prototyping. Their expertise in CNC machining, injection molding, and die casting can help you fabricate non-reactive, precisely toleranced parts to accelerate your development process.
- Stay current: Regularly review literature and regulatory updates, especially if you’re working with advanced or less-characterized oxides like copper(III) oxide or exploring new applications.
By building your workflow around trusted data, careful documentation, and expert support, you’ll reduce risk and move confidently from chemistry to manufacturable hardware—no matter how complex the oxide or application.
Copper Oxide FAQ
1. What does copper oxide do to your body?
Copper oxide exposure can irritate the eyes, respiratory, and digestive tracts. High levels may cause metal fume fever or impact liver and kidney function. Always consult the MSDS for CuO before handling and use personal protective equipment to minimize risk.
2. Is Cu2O the same as CuO?
No, Cu2O (cuprous oxide) and CuO (cupric oxide) are different compounds. Cu2O contains copper in the +1 oxidation state and is red, while CuO has copper in the +2 state and is black. Their properties and uses differ, so it's important to select the correct oxide for your application.
3. What is the common name for CuO?
CuO is commonly known as copper(II) oxide or cupric oxide. It is a black powder used in catalysts, ceramics, sensors, and as a pigment. Always specify the oxidation state to avoid confusion with other copper oxides.
4. How can I tell the difference between copper(I) oxide and copper(II) oxide in the lab?
You can distinguish them by color—Cu2O is red or reddish-brown, while CuO is black. Confirm phase identity using X-ray diffraction (XRD) or spectroscopy for reliable results, especially if purity is critical for your project.
5. What are the main industrial uses of copper oxide powders?
Cu2O is used in antifouling paints, red pigments, and solar cells, while CuO is found in catalysts, gas sensors, black ceramic glazes, and batteries. Industrial production emphasizes phase purity, safety (refer to MSDS CuO), and environmental controls.
-
Posted in
copper oxide, copper oxide applications, copper oxide properties, copper oxide synthesis, CuO vs Cu2O





