What Is Brass Made Of? Fast Answer, Properties, And Uses
What Is Brass Made Of?
Quick answer
Brass is an alloy of copper and zinc; varying the ratio changes color, strength, and workability.
What brass is in materials science
When you search for what is brass made of, the core answer is simple: brass is a copper and zinc alloy. But what does that really mean? In plain language, an alloy is a mixture of two or more metals (or a metal and another element) combined to create a new material with properties distinct from its components. In the case of brass, the main ingredients are copper (Cu) and zinc (Zn). The exact brass composition can vary, with zinc content typically ranging from about 5% up to 40% by weight, which is why there’s no single formula for what is brass metal made of. Sometimes, small amounts of other elements—like lead, tin, or silicon—are added to improve machinability, corrosion resistance, or other characteristics.
Sounds complex? Imagine a recipe where you adjust the ingredients to get different flavors. That’s how brass works: more zinc means a paler yellow color and greater strength, while less zinc leans toward a reddish hue and softer, more malleable metal. There’s no fixed recipe, which is why you’ll find many types of brass material for different uses.
Why brass matters for makers and engineers
So, why does this versatile copper and zinc alloy matter? Brass stands out for its balance of formability, strength, and resistance to corrosion. You’ll see it everywhere—from plumbing fittings and electrical connectors to musical instruments and decorative hardware. Its ability to be shaped, machined, and polished makes it a favorite for both industrial and creative projects. If you’re wondering what is brass metal made of and why it’s chosen so often, it’s because this adaptability lets manufacturers tailor brass to the needs of nearly any application.
Brass families: how copper and zinc ratios affect color and machinability
| Brass Family | Typical Copper/Zinc Ratio | Color | Machinability |
|---|---|---|---|
| Low-zinc Brass | Cu 85–95% / Zn 5–15% | Reddish-gold | Softer, very formable |
| Medium-zinc Brass | Cu 65–85% / Zn 15–35% | Yellow-gold | Balanced strength and workability |
| High-zinc Brass | Cu 55–65% / Zn 35–45% | Pale yellow | Stronger, easier to machine, less ductile |
As you move from low to high zinc content, you’ll notice not only the color of brass shifting, but also its hardness and how easily it can be cut or shaped. This is why brass is available in so many forms and grades—each designed for a particular job.
Brass, bronze, and copper: clearing up the confusion
It’s easy to mix up brass with bronze or pure copper. Here’s a quick anchor for your mental model:
- Brass: copper + zinc (mainly yellow to gold color, used for fittings and hardware)
- Bronze: copper + tin (typically reddish-brown, used for bearings and sculptures)
- Copper: nearly pure element (reddish-orange, prized for conductivity)
We’ll dig deeper into these differences in the next section, but for now, remember: brass composition is all about copper and zinc, and the ratio is what drives its unique look and performance.
Key takeaways
- Brass is a copper and zinc alloy—its properties depend on the ratio of these metals.
- There’s no single formula for what is brass made of; it’s a family of alloys with optional elements like lead or tin.
- The color, strength, and machinability of brass all shift with composition—making it highly adaptable.
- Brass is not the same as bronze or pure copper; its unique mix is why it’s so useful for plumbing, electrical, and decorative applications.
- Understanding brass material options helps you select the right type for your project, whether you need easy shaping or maximum strength.
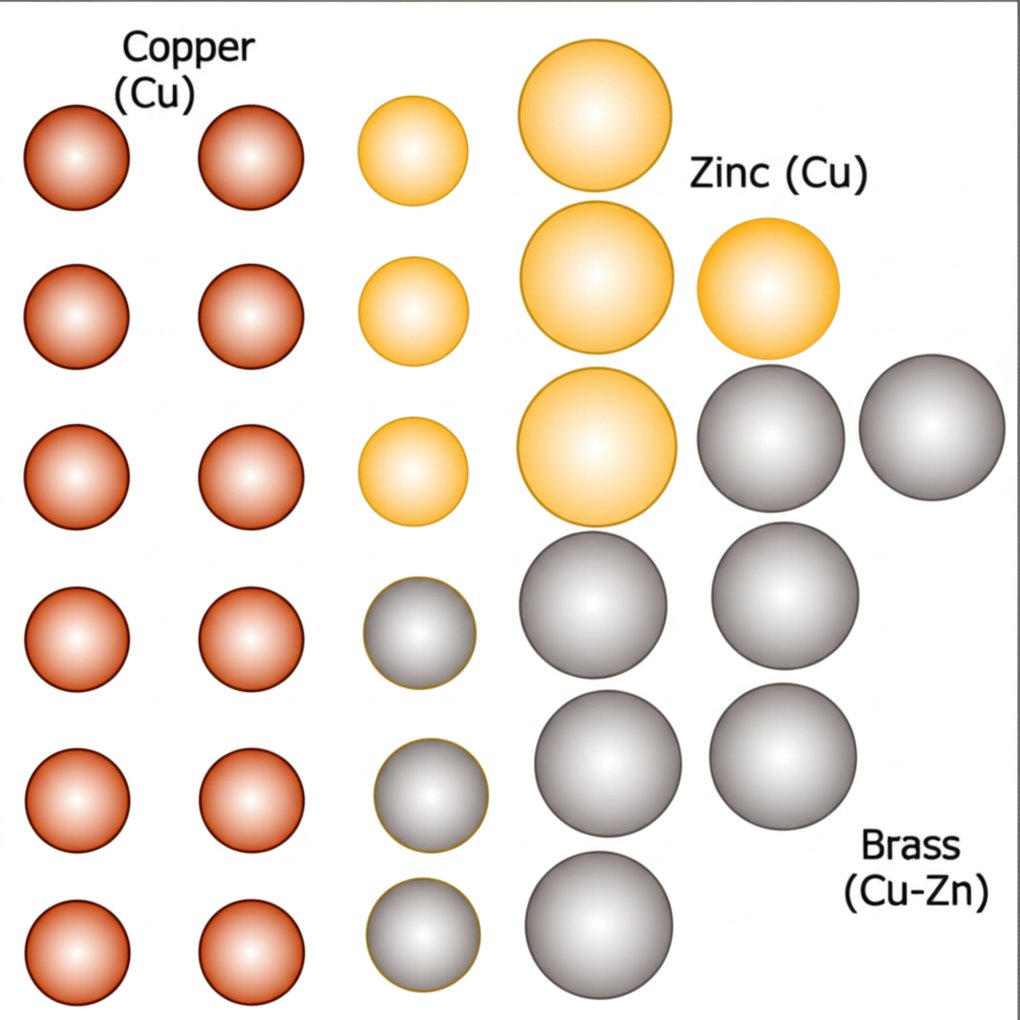
How Alloying Shapes Performance
How copper and zinc ratios shape brass alloys
Ever wondered what is brass composed of at the microscopic level? Brass is a classic example of a copper zinc alloy—a solid solution where zinc atoms substitute into the copper lattice. But the true magic of brass alloy composition lies in how much zinc is present and which other elements are added. This balance doesn’t just change the color—it transforms the way brass behaves in real-world applications.
Let’s break it down:
- Low-zinc brasses (up to ~35% zinc): These are called alpha brasses. They’re single-phase alloys, meaning the copper and zinc are thoroughly mixed at the atomic level. Alpha brasses are soft, highly ductile, and easy to cold work—think of them as the go-to for deep drawing and forming (like C260, cartridge brass).
- Medium-zinc brasses (about 32–39% zinc): Here, you’ll see a mix of alpha and beta phases. The beta phase starts to appear, boosting strength and hardness but reducing ductility. These alloys are popular for parts needing a balance of strength and workability.
- High-zinc brasses (over 39% zinc): Dominated by the beta phase, these brasses are much harder and stronger, but less formable at room temperature. They’re best for hot working and machining, not cold forming (Copper Development Association).
Common alloying additions and what they do
Brass isn’t just copper and zinc. Small tweaks—adding other metals—can make a big difference. Here’s a quick look at how additional elements shape brass alloys:
| Alloying Element | Purpose | Typical Trade-Offs |
|---|---|---|
| Lead (Pb) | Improves machinability ("free-cutting" brass) | May reduce ductility; environmental concerns |
| Tin (Sn) | Boosts corrosion resistance (e.g., for marine use) | Can slightly reduce workability |
| Aluminum (Al) | Increases strength, tarnish resistance | May alter color; harder to cold work |
| Silicon (Si) | Enhances strength, castability (used in lead-free brasses) | May affect soldering or joining |
| Nickel (Ni) | Improves toughness, gives silvery color | Higher cost, can reduce electrical conductivity |
Alpha and beta phases explained
Still sounds technical? Imagine brass as a blend of two "personalities":
- Alpha phase: Ductile, easy to shape and bend, found in lower-zinc alloys. Great for deep drawing, bending, and forming.
- Beta phase: Harder, stronger, but less bendable. Appears as zinc content rises. Suited for hot working and machining.
When both are present (alpha-beta brasses), you get a mix of properties—stronger than pure alpha, but still workable with the right processes.
Composition governs not just strength and color, but also machinability, formability, and joining behavior.
Why composition matters for real-world applications
So, brass is made of what metals? Copper, zinc, and sometimes a dash of something extra. But it’s the proportions that decide where each brass alloy shines:
- Fittings and valves: Often use brasses with added lead or silicon for machinability and corrosion resistance.
- Electrical connectors: Favor high-copper brasses for conductivity and formability.
- Decorative parts: Choose alloys for their color and polishability—nickel silver for a silvery look, red brass for a warm tone.
As you explore further, you’ll see how these choices impact not just performance, but also cost and sustainability. Next, we’ll compare brass with bronze and copper to help you spot the differences at a glance.
Brass vs Bronze vs Copper Differences That Matter
Composition at a Glance
When you’re deciding between brass vs bronze vs copper, it’s easy to get tripped up by their similar looks. But the difference between brass and bronze—and pure copper—comes down to what metals are mixed in and how those affect properties and uses. Let’s break it down visually:
| Metal | Base Elements | Common Color | Strength & Hardness | Corrosion Resistance | Electrical Conductivity | Machinability | Typical Uses | Maintenance Notes |
|---|---|---|---|---|---|---|---|---|
| Brass | Copper + Zinc (may include Pb, Sn, etc.) |
Bright yellow to gold ("brass vs bronze color" is more golden) |
Moderate; varies with zinc (stronger with more Zn) |
Good, but less than bronze (can suffer in harsh/marine settings) |
~28% of copper (medium) |
Excellent (especially free-cutting grades) |
Plumbing, fittings, musical instruments, decorative hardware | May tarnish; polish for shine; watch for dezincification in aggressive water |
| Bronze | Copper + Tin (sometimes Al, Si, Ni, P) |
Reddish-brown to dull gold ("brass vs bronze color" is deeper, more muted) |
High; hardest of the three | Excellent (best for marine/industrial use) |
~15% of copper (lower) |
Good to moderate (harder grades can be tough to machine) |
Bearings, bushings, sculptures, marine hardware | Forms protective patina; minimal upkeep; resists seawater corrosion |
| Copper | Nearly pure copper | Orange-red ("bronze vs copper"—copper is brighter and more orange) |
Lower; soft and ductile | Very good (forms greenish patina over time) |
100% (reference) (highest) |
Fair; can gum up tools | Electrical wiring, plumbing, roofing, cookware | Oxidizes to green patina (verdigris); polish for shine; easy to form |
Color and Appearance Differences
Imagine you’re holding three metal parts. How do you spot the brass vs bronze color difference? Here’s what you’ll notice:
- Brass: Usually a vibrant, yellow-gold tone—think of bright door handles or musical instruments. The higher the zinc, the paler and more golden the brass color becomes.
- Bronze: Reddish-brown to muted gold. Over time, it develops a deeper, sometimes chocolate-brown patina. Faint surface rings or a subtle matte finish are clues.
- Copper: Freshly exposed, it’s a bright orange-red. As it oxidizes, the surface darkens and eventually turns green (verdigris), especially outdoors.
But don’t rely on color alone—finishes, lacquers, and plating can disguise the true metal. For example, some "antique brass" finishes are actually bronze or even plated steel underneath.
Quick ID Cues (and Pitfalls)
- Yellowish-gold with a shiny finish? Likely brass.
- Reddish-brown or dull gold, sometimes with faint rings? Probably bronze.
- Bright orange-red, especially if a green patina forms? That’s copper.
- Watch out for: Lacquered surfaces (masking true color), electroplating, or patina treatments.
- Weight can help: copper is heaviest, bronze is heavier than brass.
- Sound test: copper is low and deep, bronze is resonant, brass is higher-pitched.
Typical Uses and Why They Differ
The difference between brass and bronze is more than just color—each metal’s composition leads to unique strengths:
- Brass is chosen for its machinability and golden appearance—ideal for precision fittings, valves, musical instruments, and decorative trim.
- Bronze stands out for toughness, wear-resistance, and corrosion resistance—making it the go-to for bushings, bearings, marine hardware, and outdoor sculptures.
- Copper is the standard for electrical and thermal conductivity—think wires, plumbing pipes, and heat exchangers.
When it comes to bronze vs copper, copper is softer and more conductive, while bronze is harder, more durable, and better in harsh environments.
Visual Cues: What to Look For
- Brass: Yellow-gold, often polished or brushed.
- Bronze: Reddish-brown, sometimes with faint surface rings or a matte finish.
- Copper: Orange-red, develops a green patina outdoors.
- Remember: Surface treatments can fool the eye—test with weight, sound, or even a small scratch (if appropriate and safe).
This guide focuses on helping you choose and process brass for your projects. If your application needs maximum wear resistance or marine performance, consider bronze. For the best electrical conductivity, copper is your answer. Next, we’ll connect these differences to the physical and mechanical properties that matter most when selecting the right metal for your job.
Key Properties
Physical Properties: Density and Melting Behavior
When you’re selecting a material for your project, understanding the density of brass and how it behaves at high temperatures is essential. So, what should you expect from this copper-zinc alloy?
| Property | Typical Value (Range) | Notes |
|---|---|---|
| Brass Density | ~8.49 g/cm³ | Varies slightly by composition and grade |
| Melting Point of Brass | 900–940°C (1650–1720°F) | Depends on zinc content; higher zinc lowers the melting temperature of brass |
| Solubility | Insoluble in water | Stable in most environments |
As you adjust the ratio of copper to zinc, the melting temperature of brass shifts, which can influence casting and joining methods. For most common brasses, the melting point of brass falls in the 900–940°C range, making it easier to cast and shape compared to pure copper or bronze alloys.
Mechanical Properties: Strength, Hardness, and Ductility
How strong is brass? The answer depends on both its composition and processing. Let’s look at some representative values for popular grades and families:
| Brass Grade / Family | Yield Strength (MPa) | Ultimate Strength (MPa) | Hardness | Ductility / Formability |
|---|---|---|---|---|
| Alpha Brass (e.g., C260 Cartridge Brass) | 124–310 | 338–469 | Moderate | Excellent (easy to cold work, deep draw) |
| Alpha-Beta Brass (e.g., C377 Forging Brass) | Higher than alpha | Higher than alpha | Higher | Good (suited for hot forging/machining) |
| Free-Cutting Brass (e.g., C360) | Varies (moderate to high) | Varies | Higher (due to lead content) | Lower (less ductile, best for machining) |
Notice how brass hardness and strength increase as you move from low-zinc, ductile brasses to high-zinc, free-cutting types. For example, cartridge brass (C260) is prized for its combination of strength and formability—ideal for deep-drawn parts. In contrast, free-machining brasses like C360 are harder and easier to machine, but less suitable for bending or drawing. Always remember: actual numbers will vary based on the exact grade, processing (cold work, annealing), and even the product form (bar, sheet, casting).
Properties are highly processing-dependent—cold work, heat treatment, and product form can shift values dramatically.
Electrical and Thermal Conductivity
Is brass conductive? Absolutely, though not as much as pure copper. Brass offers good electrical and thermal conductivity, making it suitable for electrical connectors, terminals, and heat exchanger components. As zinc content increases, conductivity drops, but it remains sufficient for many industrial and electronic uses.
- Alpha brasses (higher copper): Better conductivity, used in electrical applications.
- Higher-zinc brasses: Lower conductivity, but improved strength and machinability.
Is Brass Magnetic?
Ever tried the magnet test on a brass part? In its pure form, brass is non-magnetic. That’s because both copper and zinc are non-magnetic metals. So, is brass magnetic in any situation? Only rarely: if the alloy contains trace amounts of iron, nickel, or other magnetic elements—or if it’s been heavily cold worked—very slight magnetism may appear. But for most practical purposes, brass is considered non-magnetic.
- Non-magnetic: Ideal for sensitive electronics, instruments, and plumbing systems where magnetic interference is a concern.
- Diamagnetic: Brass can temporarily create a weak magnetic field when exposed to a strong magnet, but this effect vanishes immediately when the field is removed.
To sum up, when you’re evaluating what is brass made of for a specific project, these physical and mechanical properties help you match the right grade to the right job. Up next, we’ll explore how brass arrives in different forms and why that matters for processing and fabrication.
Brass Forms, Processing, and Joining
Common Commercial Forms and Where They Fit
Ever wonder why brass components come in so many shapes and sizes? That’s because each form is tailored for specific applications and processing needs. Here’s how the most common forms of brass material are used and what makes them unique:
- Rod and Bar: Often selected for precision-turned parts, such as valves, fasteners, and intricate machine fittings. Rods are prized for their excellent machinability—especially in free-cutting grades—making them the go-to for high-volume production of small, detailed brass components.
- Brass Sheet Metal and Plates: Used for enclosures, shims, electrical panels, and decorative trim. Sheets are favored for their formability, allowing deep drawing, bending, or stamping into complex shapes. Brass plates provide the thickness and rigidity needed for structural or load-bearing parts.
- Tubes: Essential in plumbing, heat exchangers, and condenser systems. Tubes can be seamless (drawn) or welded, and their precise tolerances make them reliable for fluid handling and thermal transfer.
- Wire: Chosen for electrical connectors, springs, pins, and jewelry. Brass wire can be drawn to fine diameters with excellent surface finish and springiness, depending on the types of brass alloyed and processing history.
- Castings: Used for complex shapes like valve bodies and decorative hardware. Casting allows the creation of near-net-shape brass components that would be costly or impossible to machine from solid stock.
Cast vs Wrought Brasses: Why Structure Matters
Here’s a practical question: when does it make sense to use cast brass instead of wrought? The answer lies in the grain structure formed during processing:
- Wrought Brasses (extruded, rolled, drawn): These have a fine, uniform grain structure, which means higher strength, better ductility, and superior machinability. Wrought forms are ideal for parts that require bending, forming, or tight tolerances—think of brass sheet metal for deep drawing or rods for turned parts.
- Cast Brasses: Produced by pouring molten brass into molds. Castings can achieve intricate shapes and internal features but typically have a coarser grain structure, making them less ductile and sometimes less strong than wrought forms. Cast brass is best for large, complex, or low-volume parts where machining would be wasteful or impractical.
You’ll notice that wrought brass parts are usually denser and smoother, while cast brass can sometimes show minor surface imperfections or be lighter if hollow. This difference impacts both performance and appearance.
Forming, Joining, and Finishing Tips
Once you’ve chosen the right form, how you process and join brass makes all the difference:
- Cold Working: Bending, drawing, or rolling can strengthen brass but may reduce ductility. For deep forming, use single-phase (alpha) brasses; for machining, choose free-cutting types. After heavy cold work, annealing (controlled heating) can relieve stresses and restore softness.
- Joining: Brass is easily soldered or brazed thanks to its good wetting behavior. For strong, leak-tight joints in plumbing or electronics, design for proper fit and avoid overheating, which can affect grain structure or cause zinc loss. Welding is less common—some brasses can emit zinc fumes or become brittle if not handled carefully.
- Finishes: Polishing, brushing, and lacquering are popular ways to enhance the look and durability of brass. For best results, always clean and prepare the surface thoroughly before applying coatings. Plating (chrome, nickel, gold) is also common for added protection or decorative effect.
When you’re planning a project, matching the types of brass and their processing routes to your end-use ensures your parts perform as expected—whether you need a brilliant finish, precise fit, or robust corrosion resistance. Next, we’ll dive into corrosion and how to keep your brass looking and working its best.
Does Brass Rust and How to Protect It?
Does Brass Rust and What Really Happens?
When you’re choosing materials for plumbing, marine, or decorative projects, you might wonder: does brass rust? The short answer is no—brass does not rust in the way iron or steel does. Rust is a specific type of corrosion that affects iron-containing metals, resulting in flaky, reddish-brown iron oxide. Since brass is made of copper and zinc, with no iron in its makeup, do brass rust or does brass metal rust are answered with a clear “no”.
But that doesn’t mean brass is immune to all forms of corrosion. Instead of rust, brass undergoes different surface changes:
- Tarnish: A dulling of the surface caused by oxidation of copper, often leaving a brown or black film.
- Patina: A greenish or blue-green layer (mainly copper carbonate) that forms over time, especially outdoors. This patina can actually protect the underlying metal from further damage.
- Dezincification: In certain environments, especially with water containing chlorides or low pH, zinc can be selectively leached from the brass, leaving behind a weak, porous, copper-rich structure.
Understanding Dezincification
Dezincification is the most serious corrosion threat to brass, especially in plumbing, marine, and industrial systems. It happens when water or moist environments cause the zinc in brass to dissolve out, leaving a spongy matrix of copper. This process can be slow and subtle, but over time, it can lead to leaks, failures, or even catastrophic bursts in pipes and fittings (Inspenet).
There are two main types of dezincification:
- Uniform dezincification: A widespread, shallow loss of zinc across the surface, often making the brass look pinkish or reddish instead of its usual yellow.
- Localized dezincification: Deep, patchy attack in specific spots, leading to pitting, cavities, and critical weakness—especially dangerous in pressurized systems.
Brasses with higher zinc content are generally more susceptible to dezincification. However, specialized brasses—often containing small amounts of tin, arsenic, or phosphorus—are engineered to resist this process. If your application involves aggressive water or marine conditions, specifying dezincification-resistant brass (DZR) is a smart move (Canadian Conservation Institute).
How to Recognize and Prevent Damage
Early detection of corrosion or dezincification can save you from costly repairs. Watch for these warning signs:
- Color changes: Areas turning pink or red indicate zinc loss.
- Porous or crumbly texture: The surface feels weak or spongy.
- Leaks or pressure drops: Especially in plumbing, these may signal internal corrosion.
- Surface pitting or flaking: Advanced dezincification can cause visible cavities or even perforation.
If you spot these symptoms, act quickly—replacement of affected parts is usually the safest solution, as the damage is not reversible.
Practical Strategies for Corrosion and Dezincification Prevention
- Material selection: Choose dezincification-resistant brasses (often with tin, arsenic, or phosphorus) for plumbing, marine, or harsh environments.
- Protective coatings and plating: Apply clear lacquer, wax, or specialized coatings to block corrosive agents.
- Water chemistry control: Monitor and adjust pH, minimize chlorides, and avoid stagnant water in systems.
- Design for drainage: Prevent water from pooling in fittings or joints.
- Use of corrosion inhibitors: In industrial systems, these additives can slow the corrosion process.
- Regular inspection and maintenance: Check for early signs of corrosion and replace compromised parts promptly.
Match brass type to the service environment; aggressive waters can demand dezincification-resistant compositions or alternative alloys.
To sum up, while the answer to "do brass rust" is no, brass can corrode—especially through dezincification in challenging environments. By understanding the risks and taking proactive steps, you’ll extend the life and reliability of your brass components. Next, we’ll explore how to identify and choose the right brass for your specific needs.

Identify and Choose the Right Brass
DIY Identification Steps: How to Tell the Difference Between Copper and Brass (and Bronze)
Ever picked up a mystery metal part and wondered, "How do you tell the difference between brass and copper?" Or maybe you’re sorting scrap and need to know, "How can I tell the difference between copper and brass, or even bronze?" Here’s a hands-on guide to help you spot brass confidently—at home, in the workshop, or on the job site.
-
Visual Inspection
- Brass color: Look for a bright yellow-gold hue. The higher the zinc content, the paler and more golden the color of brass becomes. Copper is distinctly orange-red, while bronze is more reddish-brown or muted gold.
- Check for plating or lacquer—these can mask the true color. Scratches or worn spots may reveal the base metal underneath.
- Ask yourself: "What color is brass compared to copper or bronze?" If it’s yellowish and shiny, it’s likely brass.
-
Magnet Test (Brass Magnetism)
- Pure brass is non-magnetic. Hold a magnet to the object; if it sticks, the piece is likely steel or iron, not brass. If there’s no attraction, it could be brass, copper, or bronze.
- Note: Some impurities or heavy cold work can cause slight magnetism, but generally, brass magnetism is negligible.
-
Weight and Feel
- Brass is lighter than copper but heavier than many steels. If you have a similar-sized copper and brass piece, the copper will feel denser.
- Bronze is usually heavier than brass, but the difference can be subtle.
-
Scratch Test (Use Caution!)
- In an inconspicuous spot, gently scratch the surface. Brass is softer than bronze but harder than pure copper. If the mark is bright yellow, it’s likely brass; reddish-orange suggests copper.
- Always use PPE and avoid inhaling dust, especially with old or unknown alloys—some brasses contain lead.
-
Patina and Surface Changes
- Brass develops a brown or dark tarnish; copper forms a greenish patina (verdigris) over time. Bronze may darken to a chocolate brown.
- Environmental exposure, finishes, or contamination can alter the appearance, so use this clue in combination with others.
-
Limitations and Safety Notes
- Surface coatings, heat tint, or contamination can mislead visual and scratch tests.
- Never conduct destructive tests on valuable or historic items. When in doubt, consult a professional or use XRF analysis for precise alloy ID.
- Always wear gloves and a dust mask when handling or testing unknown metals—lead and other additives can pose health risks.
Practical Selection by Application: Choosing the Best Brass Alloy
Once you’ve identified your material, the next step is matching the right type of brass to your application. Here’s a quick guide to help you balance machinability, corrosion resistance, and aesthetics—because what is brass made of directly impacts performance and appearance.
-
Plumbing and Water Systems
- Look for brasses with high corrosion and dezincification resistance (often with tin or special additives).
- Ensure regulatory suitability—some brasses contain lead, which may be restricted in potable water systems.
- Trade-off: Higher resistance alloys may be less machinable or more expensive.
-
Electrical Connectors and Terminals
- Prioritize conductivity (higher copper content) and stress relaxation resistance.
- Trade-off: Alloys with more copper are softer and may wear faster in high-stress applications.
-
Musical Instruments
- Choose brasses with excellent formability and consistent brass color for polishing and finishing.
- Trade-off: Softer alloys are easier to shape but may dent more easily.
-
Fittings, Fasteners, and Machined Parts
- Select free-machining brasses (often with lead) for efficiency and dimensional stability.
- Trade-off: Leaded brasses may not meet all health or environmental regulations.
-
Decorative and Architectural Uses
- Pick alloys with a bright, golden color of brass and good polishability—C260 (cartridge brass) is a popular choice.
- Trade-off: Surface finish may require regular maintenance to retain brilliance.
Trade-Offs to Consider: Balancing Machinability, Formability, and Corrosion Resistance
Imagine you’re planning a project and need to decide: Should you choose a brass that’s easy to machine or one that’s more resistant to corrosion? Here’s how to weigh your options:
- Machinability vs. Corrosion Resistance: Free-machining brasses (with lead) are great for complex parts but may not be ideal for drinking water or marine environments.
- Formability vs. Strength: High-copper, low-zinc brasses bend and form easily but may lack the strength of higher-zinc alloys.
- Appearance vs. Durability: Alloys chosen for their bright brass color may tarnish faster or require more upkeep.
For critical or regulated applications, always consult material datasheets and relevant standards. If you’re ever unsure, seek expert advice or laboratory analysis to confirm your brass alloy’s suitability.
Understanding these identification steps and selection criteria ensures you get the right performance, safety, and look for your application. Up next, we’ll demystify brass grades, standards, and how to cross-reference them for your next project.
Brass Grades, Standards, and Cross References
How Brass Grades Are Named
When you’re choosing a brass alloy for your project, you’ll quickly notice a maze of grade names, numbers, and standards. Sounds confusing? Think of it as a translation guide for the composition of brass across industries and regions. Each system—UNS (Unified Numbering System), ASTM (American Society for Testing and Materials), and EN (European Norm)—labels brass grades based on their brass metal composition and intended use. For example, “C26000” (UNS) is also known as “Cartridge Brass” and might be referenced as “CuZn30” in EN standards.
UNS, ASTM, and EN Mapping
Let’s make sense of the naming systems with a cross-reference table. This helps you find equivalent grades whether you’re reading a U.S. datasheet or a European spec sheet. The following table is based on industry references and conversion charts:
| Common Name | UNS Number | ASTM Spec | EN Designation | Notes |
|---|---|---|---|---|
| Cartridge Brass | C26000 | B36 | CuZn30, CW505L | High formability, deep drawing |
| Muntz Metal | C28000 | B111 | CuZn40, CW509L | Hot working, marine fittings |
| Free Machining Brass | C36000 | B16 | CuZn36Pb3, CW603N | Excellent machinability |
| Architectural Bronze | C38500 | B455 | CuZn39Pb3, CW614N | Extruded shapes, good machinability |
| Naval Brass | C46400 | B21 | CuZn39Sn1, CW712R | Marine, corrosion resistant |
These mappings are a starting point—always check the full standard or supplier certificate for critical applications, as subtle differences in brass material composition can impact performance.
Typical Brass Composition Ranges and Uses by Family
Brass isn’t a single recipe—it’s a family of alloys. Here’s a table summarizing common families, their brass metal composition (by weight), and where you’ll find them in action, based on available reference data:
| Brass Family / Grade | Typical Composition (Cu/Zn/Other) | Key Properties | Common Uses |
|---|---|---|---|
| Alpha Brass (e.g., C26000) | Cu 70% / Zn 30% | Excellent formability, ductile | Cartridges, deep-drawn parts, musical instruments |
| Alpha-Beta Brass (e.g., C28000) | Cu 60% / Zn 40% | Stronger, suited for hot work | Marine fittings, condenser tubes |
| Free Machining Brass (e.g., C36000) | Cu 60% / Zn 35.5% / Pb up to 3.7% | 100% machinability, fair formability | Fittings, fasteners, valves, hardware |
| Naval Brass (e.g., C46400) | Cu 59% / Zn 40% / Sn 1% | High corrosion resistance | Marine hardware, propellers, pumps |
| Architectural Bronze (e.g., C38500) | Cu 59% / Zn 42% / Pb up to 3.5% | Easy to form and machine | Extrusions, decorative trim |
Keep in mind: The composition of brass within each family can shift slightly depending on the supplier and product form. Always verify with a datasheet or test certificate for mission-critical jobs.
Glossary: Key Brass Terms Explained
- Alpha phase: Single-phase solid solution of zinc in copper; soft, ductile, easily formed.
- Beta phase: Harder, stronger phase appearing as zinc content rises; less ductile, suited for hot working.
- Alpha–beta: Brass containing both phases; balances strength and workability.
- Dezincification: Selective leaching of zinc from brass, weakening the alloy (especially in plumbing).
- Annealing: Controlled heating to restore softness and relieve stress after cold working.
- Stress relaxation: Loss of clamping force or springiness over time under load, relevant for electrical connectors.
Where to Find Authoritative Data
For the most reliable information on what metals are in brass and how to specify the right grade, consult these trusted sources:
- ASTM International: Full brass and copper alloy standards (product specs, test methods)
- ISO and CEN: International and European standards for composition and product forms
- MatWeb: Database of material properties and brass metal made of data
- ASM Handbooks: In-depth technical references, alloy data, and application guidance
- Supplier datasheets and certificates: Always request these for exact brass material composition on critical projects
Remember, values and designations may evolve with new standards and improved processes. For safety, compliance, and performance, defer to the governing standard and supplier certification every time.
With a clear understanding of brass grades and standards, you’ll be ready to make informed choices—whether you’re ordering parts, specifying materials, or troubleshooting a project. Next, we’ll cover safety, compliance, and responsible handling of brass alloys in real-world applications.
Safety, Compliance, and Responsible Use
Lead and Other Alloying Elements: Why It Matters for Handling
When you’re working with brass, you might ask: is brass a homogeneous mixture, or is it a compound? Brass is a homogeneous mixture—an alloy—of copper, zinc, and sometimes other elements like lead, tin, or silicon. Lead is often added to certain brasses to create “free-machining” alloys, which makes them much easier to cut and shape. But this convenience comes with a trade-off: lead can pose health and environmental risks, especially when brass parts are used in drinking water systems or when you’re handling chips and dust during machining.
Imagine you’re machining a brass rod for plumbing. If it’s a leaded alloy, the chips and dust can contain lead particles. These particles can be inhaled or absorbed through the skin, so proper precautions are essential. In some regions, the use of leaded brasses is restricted or even banned for potable water applications. Always check the specific alloy’s datasheet and local regulations before selecting a brass for projects involving water, food, or skin contact.
Potable Water and Contact Safety: Know Your Regulations
Thinking about using brass for faucets, valves, or any part that touches drinking water? Regulations have become much stricter in recent years. In the United States, the Safe Drinking Water Act (SDWA) limits the allowable lead content in the wetted surfaces of plumbing products to a weighted average of 0.25%. This means that not all brass alloys are automatically suitable for potable water—only those certified as “lead-free” under NSF/ANSI standards can be used legally and safely (EPA). Canada and other countries have similar requirements.
Why so strict? Even low levels of lead exposure can have serious health effects, especially for children and pregnant women. Lead can leach from brass fittings into drinking water, particularly if the water is acidic or sits in pipes for extended periods. That’s why it’s critical to:
- Specify only certified lead-free brass for potable water systems.
- Consult local codes and standards (such as NSF 61/372 in North America) before installation.
- Request supplier documentation and third-party certifications for all wetted parts.
If you’re unsure, contact your local water authority or review the latest EPA and NSF guidelines for up-to-date requirements.
Responsible Handling and Recycling: Shop Safety and Environmental Best Practices
Is brass a mixture or compound? As a mixture, its properties and risks depend on what’s inside—and that means safety practices matter. Whether you’re machining, recycling, or disposing of brass, here’s how to protect yourself and the environment:
- Wear PPE: Always use gloves, safety glasses, and a dust mask when cutting, grinding, or handling brass—especially if it may contain lead.
- Ventilation: Work in a well-ventilated area or use local exhaust systems to reduce dust and fume exposure.
- Chip Management: Collect and dispose of brass chips and dust safely. Keep leaded and unleaded scrap separate—this helps recyclers avoid contamination and ensures regulatory compliance.
- Hygiene: Wash hands after handling brass, especially before eating or drinking. Avoid bringing work clothes home to prevent lead dust exposure to family members.
- Recycling: Brass is 100% recyclable, but proper sorting is essential. Identify alloys (leaded vs. lead-free) and communicate with your recycler to ensure safe, responsible processing.
Always verify alloy certification and regulatory compliance with your supplier when parts contact drinking water or food.
To sum up: Is brass a compound? No, it’s a homogeneous mixture with properties—and responsibilities—that depend on its exact composition. By staying informed and following best practices, you’ll keep your projects safe, compliant, and sustainable. Up next, we’ll explore how to machine brass for precision parts and when to seek expert support for demanding applications.

Machining Brass Materials
Machinability Tips for Brass
When you look at what is brass made of, it’s easy to see why this alloy is beloved in the machine shop. Brass’s copper-zinc foundation (sometimes with a dash of lead) gives it a rare combination of softness, ductility, and chip-breaking behavior. That means you can cut, turn, and mill brass faster and with less tool wear than most other metals. But even with these advantages, smart machining makes all the difference—especially if you want flawless parts, tight tolerances, and a professional finish.
- Use sharp carbide tools: Brass’s low friction means sharp carbide inserts or end mills last longer and produce crisp edges. Dull tools can smear the surface or cause burrs, especially with softer brass alloys.
- Opt for a positive rake angle: This helps the tool slice cleanly, reducing cutting forces and heat. Positive rake is especially helpful for free-cutting brasses like C360.
- Control your speeds and feeds: Brass supports higher cutting speeds than steel or aluminum. For C360, spindle speeds in the 80–150 SFM (surface feet per minute) range are common, but always adjust based on your specific alloy and tool setup.
- Apply appropriate coolant: Brass doesn’t work-harden much, but heat can still build up—especially at high speeds. Use cutting fluids or air cooling to prevent surface oxidation and maintain tight tolerances.
- Secure workholding for thin brass sheet: Brass sheet metal is easily deformed. Use soft jaws, vacuum chucks, or sacrificial backing to prevent distortion during cutting or drilling.
- Deburring is essential: Despite its excellent machinability, brass can leave behind small burrs, especially on drilled or milled edges. Plan for a final deburring or polishing step to ensure safe, functional parts.
Tolerances and Surface Finish: Getting Precision Right
One of the standout advantages of brass materials is their ability to hold tight tolerances and deliver a beautiful finish with minimal effort. Whether you’re making electrical connectors, plumbing fittings, or decorative hardware, here’s what you need to know:
- Dimensional control: Brass is stable and resists warping, so tolerances as tight as ±0.001" (0.025 mm) are achievable with the right equipment. For ultra-precision parts—like aerospace connectors—tolerances down to ±0.0005" (0.0127 mm) are possible.
- Surface finish options: Brass can be polished to a high gloss, brushed for a satin look, or left with a clean as-machined finish. After machining, common finishing techniques include:
- Polishing: Removes tool marks, enhances shine, and is perfect for decorative brass metal alloy parts.
- Buffing and honing: Refines the surface texture, delivering a mirror-like finish for high-end applications.
- Powder coating or electroplating: Adds corrosion resistance and durability—great for outdoor or harsh environments.
- Sandblasting: Creates a matte or textured finish, ideal for parts that need extra grip or a rugged appearance.
- Protective lacquers: Seal the surface and prevent tarnish or patina formation on visible brass components.
- Avoid abrasive grit: When finishing, ensure that no abrasive particles are embedded in the brass surface. This can cause premature wear or interfere with the part’s function.
Brass’s free-cutting grades enable high productivity, but verify lead content and end-use requirements early.
From Prototype to Production: When to Involve a Specialist
Ready to move from a single prototype to a production run of hundreds or thousands of brass components? Here’s where the right partner and process can make or break your project:
- Complex geometries: If your design features intricate curves, deep pockets, or undercuts, 4- or 5-axis CNC machining is essential for accuracy and repeatability.
- Fast iteration cycles: Rapid prototyping with brass allows you to test fit, function, and finish—then tweak your design before scaling up.
- Tight tolerances for regulated industries: Aerospace, medical, and electronics sectors demand precision and certified quality. Look for ISO-certified shops with experience in your field.
- Material selection and traceability: Always confirm the alloy type (C360, C464, etc.) and require supplier certification, especially if your uses of brass include potable water, food contact, or safety-critical applications.
- When to involve a specialist: If your project requires ultra-tight tolerances, complex features, or rapid delivery, partnering with a precision CNC shop makes sense. For example, XTJ CNC Machining Services offers 4- and 5-axis capacity, fast turnarounds, and ISO 9001:2015/IATF16949 certifications—ensuring your brass parts meet both performance and regulatory standards.
Brass’s machinability and formability make it a go-to choice for everything from custom prototypes to mass-produced fittings. By focusing on best practices in machining, finish, and material selection—and knowing when to call in the experts—you’ll unlock the full potential of this versatile brass metal alloy for your next project.
Frequently Asked Questions About Brass
1. Why is brass so cheap?
Brass is typically less expensive than many other metals because it is made primarily from copper and zinc, which are more affordable and widely available. Its production process is also efficient, making brass a cost-effective choice for many applications.
2. What brass is worth money?
Brass types like yellow brass (used in plumbing fixtures), red brass (found in musical instruments), and cast brass (used in decorative items) are valuable as scrap. Their worth depends on purity, composition, and current metal market prices.
3. Does brass rust?
Brass does not rust like iron or steel because it contains no iron. Instead, it can tarnish or develop a patina and, in certain environments, may experience dezincification, which can weaken the material if not addressed.
4. What is the difference between brass and bronze?
Brass is an alloy of copper and zinc, usually golden yellow, while bronze is mainly copper and tin, with a reddish-brown color. Brass is favored for its machinability and appearance, while bronze excels in strength and corrosion resistance, especially in marine environments.
5. How can I tell if an item is brass or copper?
Check the color: brass is yellow-gold, while copper is orange-red. Brass is non-magnetic and lighter than copper. You can also perform a scratch test in an inconspicuous area—brass reveals a yellow mark, copper shows reddish-orange. For valuable or historical items, consult a professional.





